
PDF Publication Title:
Text from PDF Page: 003
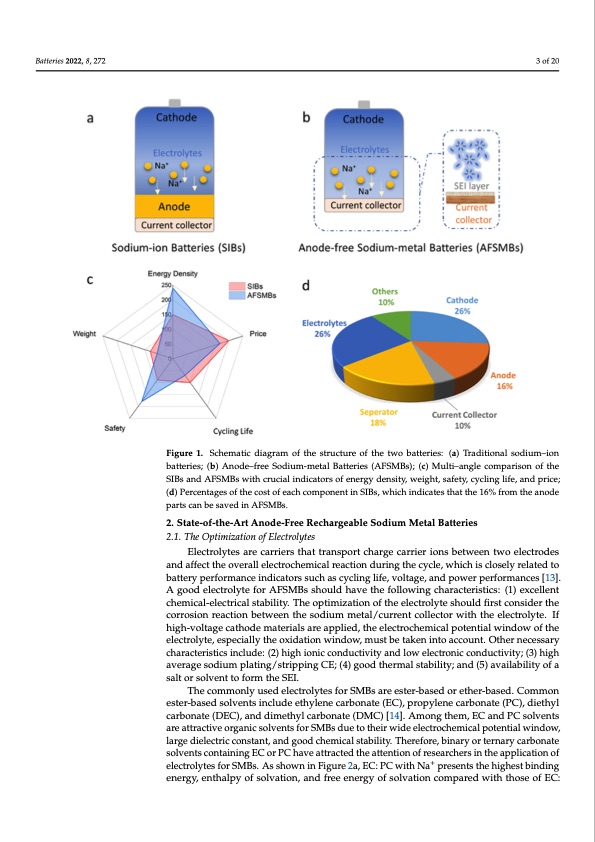
Batteries 2022, 8, x FOR PEER REVIEW 3 of 19 3 of 20 Figure 1. Schematic diagram of the structure of the two batteries: (a) Traditional sodium–ion Figure 1. Schematic diagram of the structure of the two batteries: (a) Traditional sodium−ion batter- batteries; (b) Anode–free Sodium-metal Batteries (AFSMBs); (c) Multi–angle comparison of the ies; (b) Anode−free Sodium-metal Batteries (AFSMBs); (c) Multi−angle comparison of the SIBs and AFSIMBsBasnwd iAthFScMruBcsiawl inthdcicruatcoiarlsionfdeicnaetorgrsyodfenseirtgyy, wdenigsihtyt, swaefeigthyt,,csyacfleitnyg, clyifceli,nagndlifpe,raicned; (pdr)icPee; rcent- age(ds)oPfetrhcenctaogsetsooffetahcehcocsotmofpeoanchenctominpSoInBesn,twinhSicIBhsi,nwdhiiccahteinsdtihcatetshteha1t6t%hef1r6o%mftrhome atnheodaneopdaerts can bepsarvtsedcainbAeFsaSvMedBsin. AFSMBs. 2. State-of-the-Art Anode-Free Rechargeable Sodium Metal Batteries Batteries 2022, 8, 272 Future research should focus on developing the electrolyte matching with the 2.1. The Optimization of Electrolytes AFSMBs, stabilizing the electrochemical interfaces, and developing the appropriate so- Electrolytes are carriers that transport charge carrier ions between two electrodes dium metal deposition current collectors to improve the performance of AFSMBs. How- and affect the overall electrochemical reaction during the cycle, which is closely related to ever, there is still a lack of understanding and recognition of these aspects. In this review, battery performance indicators such as cycling life, voltage, and power performances [13]. weAregvoiosditedlectthreolmytielefsotroAneFSaMchBisevshemoueldnthsaovfeAthFeSMfoBllos,winincglucdhianrgac:t(eir)itshtiecso:p(t1i)meixzcaetllieontof the elecchtermoliyctaels-e;l(eici)trtihcael cstoanbsiltirtuy.cTtihoenoopftiamnizeaffticoinenoft tShEeIelaeycterro;lyatnedsh(ioiui)ldthfiersmt ocodnifsicdaetriothneof the corrosion reaction between the sodium metal/current collector with the electrolyte. If current collectors to regulate sodium deposition. In the end, we put forward the chal- high-voltage cathode materials are applied, the electrochemical potential window of the lenges and future perspectives regarding the fabrication of AFSMBs. electrolyte, especially the oxidation window, must be taken into account. Other necessary characteristics include: (2) high ionic conductivity and low electronic conductivity; (3) high 2. State-of-the-Art Anode-Free Rechargeable Sodium Metal Batteries average sodium plating/stripping CE; (4) good thermal stability; and (5) availability of a 2.1. The Optimization of Electrolytes salt or solvent to form the SEI. The commonly used electrolytes for SMBs are ester-based or ether-based. Common Electrolytes are carriers that transport charge carrier ions between two electrodes and ester-based solvents include ethylene carbonate (EC), propylene carbonate (PC), diethyl affect the overall electrochemical reaction during the cycle, which is closely related to bat- carbonate (DEC), and dimethyl carbonate (DMC) [14]. Among them, EC and PC solvents tery performance indicators such as cycling life, voltage, and power performances [13]. A are attractive organic solvents for SMBs due to their wide electrochemical potential window, good electrolyte for AFSMBs should have the following characteristics: (1) excellent chem- large dielectric constant, and good chemical stability. Therefore, binary or ternary carbonate ical-electrical stability. The optimization of the electrolyte should first consider the corro- solvents containing EC or PC have attracted the attention of researchers in the application of sion reaction between the sodium metal/current collector with the electrolyte. If high-volt- electrolytes for SMBs. As shown in Figure 2a, EC: PC with Na+ presents the highest binding agenceartghyo, denetmhaalpteyrioaflsoalrveaatipopn,liaendd, tfhreeeelencetrgoychoef msoilcvaltipoontecnomtiaplawreidndwoitwh othfothseoefleEcCtr:olyte, especially the oxidation window, must be taken into account. Other necessary character- istics include: (2) high ionic conductivity and low electronic conductivity; (3) high average sodium plating/stripping CE; (4) good thermal stability; and (5) availability of a salt or solvent to form the SEI.PDF Image | Anode-Free Rechargeable Sodium-Metal Batteries
PDF Search Title:
Anode-Free Rechargeable Sodium-Metal BatteriesOriginal File Name Searched:
batteries-08-00272.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |