
PDF Publication Title:
Text from PDF Page: 007
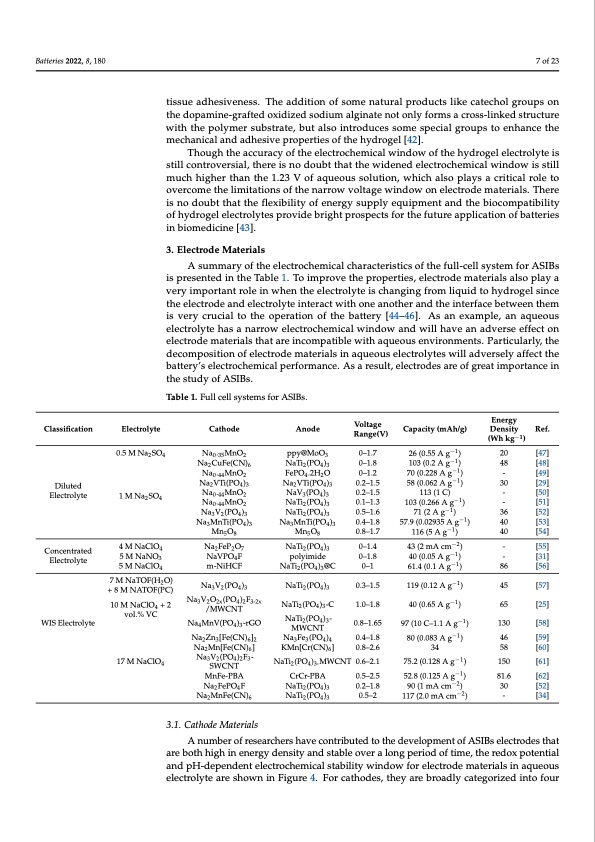
Batteries 2022, 8, 180 7 of 23 tissue adhesiveness. The addition of some natural products like catechol groups on the dopamine-grafted oxidized sodium alginate not only forms a cross-linked structure with the polymer substrate, but also introduces some special groups to enhance the mechanical and adhesive properties of the hydrogel [42]. Though the accuracy of the electrochemical window of the hydrogel electrolyte is still controversial, there is no doubt that the widened electrochemical window is still much higher than the 1.23 V of aqueous solution, which also plays a critical role to overcome the limitations of the narrow voltage window on electrode materials. There is no doubt that the flexibility of energy supply equipment and the biocompatibility of hydrogel electrolytes provide bright prospects for the future application of batteries in biomedicine [43]. 3. Electrode Materials A summary of the electrochemical characteristics of the full-cell system for ASIBs is presented in the Table 1. To improve the properties, electrode materials also play a very important role in when the electrolyte is changing from liquid to hydrogel since the electrode and electrolyte interact with one another and the interface between them is very crucial to the operation of the battery [44–46]. As an example, an aqueous electrolyte has a narrow electrochemical window and will have an adverse effect on electrode materials that are incompatible with aqueous environments. Particularly, the decomposition of electrode materials in aqueous electrolytes will adversely affect the battery’s electrochemical performance. As a result, electrodes are of great importance in the study of ASIBs. Table 1. Full cell systems for ASIBs. Classification Diluted Electrolyte Concentrated Electrolyte WIS Electrolyte Electrolyte 0.5 M Na2SO4 1 M Na2SO4 4 M NaClO4 5 M NaNO3 5 M NaClO4 7 M NaTOF(H2O) + 8 M NATOF(PC) 10 M NaClO4 + 2 vol.% VC 17 M NaClO4 Cathode Na0.35MnO2 Na2CuFe(CN)6 Na0 .44 MnO2 Na2 VTi(PO4 )3 Na0 .44 MnO2 Na0 .44 MnO2 Na3 V2 (PO4 )3 Na3 MnTi(PO4 )3 Mn5 O8 Na2FeP2O7 NaVPO4F m-NiHCF Na3V2(PO4)3 Na3V2O2x(PO4)2F3-2x /MWCNT Na4MnV(PO4)3-rGO Na2 Zn3 [Fe(CN)6 ]2 Na2 Mn[Fe(CN)6 ] Na3 V2 (PO4 )2 F3 - SWCNT MnFe-PBA Na2 FePO4 F Na2MnFe(CN)6 Voltage Anode Range(V) ppy@MoO3 0–1.7 NaTi2(PO4)3 0–1.8 FePO4 .2H2 O 0–1.2 Na2 VTi(PO4 )3 0.2–1.5 NaV3 (PO4 )3 0.2–1.5 NaTi2 (PO4 )3 0.1–1.3 NaTi2 (PO4 )3 0.5–1.6 Na3 MnTi(PO4 )3 0.4–1.8 Mn5 O8 0.8–1.7 NaTi2(PO4)3 0–1.4 polyimide 0–1.8 NaTi2(PO4)3@C 0–1 NaTi2(PO4)3 0.3–1.5 NaTi2(PO4)3-C 1.0–1.8 NaTi2(PO4)3- 0.8–1.65 MWCNT Na3 Fe3 (PO4 )4 0.4–1.8 KMn[Cr(CN)6 ] 0.8–2.6 NaTi2 (PO4 )3- MWCNT 0.6–2.1 CrCr-PBA 0.5–2.5 NaTi2 (PO4 )3 0.2–1.8 NaTi2(PO4)3 0.5–2 Capacity (mAh/g) 26 (0.55 A g−1) 103 (0.2 A g−1) 70 (0.228 A g−1 ) 58 (0.062 A g−1 ) 113 (1 C) 103 (0.266 A g−1) 71 (2 A g−1) 57.9 (0.02935 A g−1) 116 (5 A g−1) 43 (2 mA cm−2 ) 40 (0.05 A g−1) 61.4 (0.1 A g−1 ) 119 (0.12 A g−1 ) 40 (0.65 A g−1) 97 (10 C–1.1 A g−1) 80 (0.083 A g−1) 34 75.2 (0.128 A g−1) 52.8 (0.125 A g−1) 90 (1 mA cm−2) 117 (2.0 mA cm−2) Energy Density Ref. (Wh kg−1) 20 [47] 48 [48] - [49] 30 [29] - [50] - [51] 36 [52] 40 [53] 40 [54] - [55] - [31] 86 [56] 45 [57] 65 [25] 130 [58] 46 [59] 58 [60] 150 [61] 81.6 [62] 30 [52] - [34] 3.1. Cathode Materials A number of researchers have contributed to the development of ASIBs electrodes that are both high in energy density and stable over a long period of time, the redox potential and pH-dependent electrochemical stability window for electrode materials in aqueous electrolyte are shown in Figure 4. For cathodes, they are broadly categorized into fourPDF Image | Aqueous Rechargeable Sodium-Ion Batteries Hydrogel
PDF Search Title:
Aqueous Rechargeable Sodium-Ion Batteries HydrogelOriginal File Name Searched:
batteries-08-00180-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |