
PDF Publication Title:
Text from PDF Page: 008
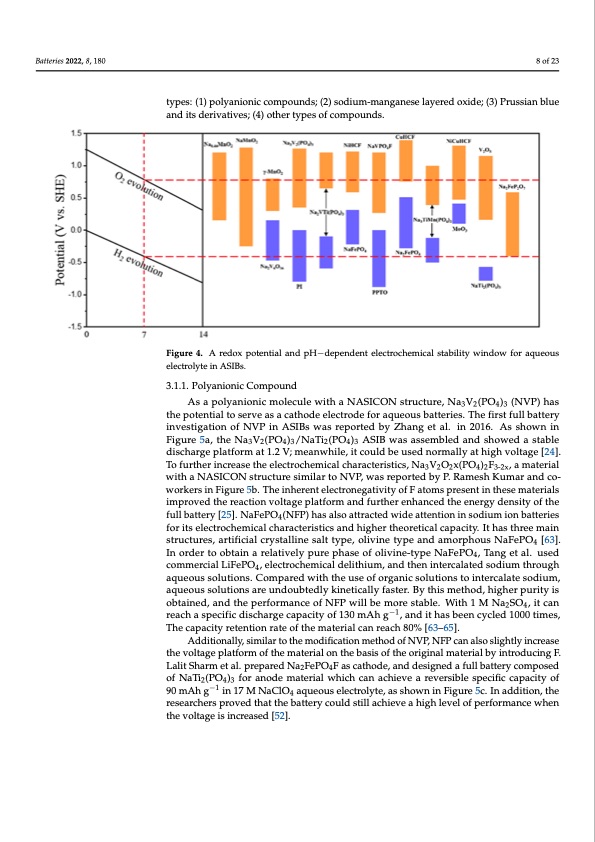
Batteries 2022, 8, 180 A number of researchers have contributed to the development of ASIBs elect that are both high in energy density and stable over a long period of time, the redo tential and pH-dependent electrochemical stability window for electrode materi aqueous electrolyte are shown in Figure 4. For cathodes, they are broadly c8 aoft2e3gorize four types: (1) polyanionic compounds; (2) sodium-manganese layered oxide; (3) Pru blue and its derivatives; (4) other types of compounds. types: (1) polyanionic compounds; (2) sodium-manganese layered oxide; (3) Prussian blue and its derivatives; (4) other types of compounds. Figure 4. A redox potential and pH−dependent electrochemical stability window for aqueous electrolyte in ASIBs. Figure 4. A redox potential and pH−dependent electrochemical stability window for aqueou trolyte in ASIBs. 3.1.1. Polyanionic Compound As a polyanionic molecule with a NASICON structure, Na3V2(PO4)3 (NVP) has 3.1.1. PthoelypoatneinotinailctoCsoermvepaosuancadthode electrode for aqueous batteries. The first full battery investigation of NVP in ASIBs was reported by Zhang et al. in 2016. As shown in As a polyanionic molecule with a NASICON structure, Na3V2(PO4)3 (NVP) h Figure 5a, the Na3V2(PO4)3/NaTi2(PO4)3 ASIB was assembled and showed a stable potentdiiaslchtoarsgerpvlaetfaosrma acta1th.2oVd; emelaencwtrhoilde,eitfcoorualdqbueuosuesd bnaortmtearlileysa.tThhigehfvirosltafguel[l2b4]a. ttery i To further increase the electrochemical characteristics, Na V O x(PO ) F , a material tigation of NVP in ASIBs was reported by Zhang et al3. i2n 2016.4A2s3s-2hxown in Figure 5 with a NASICON structure similar to NVP, was reported by P. Ramesh Kumar and co- Na3V2(PO4)3/NaTi2(PO4)3 ASIB was assembled and showed a stable discharge platfo workers in Figure 5b. The inherent electronegativity of F atoms present in these materials 1.2 V; meanwhile, it could be used normally at high voltage [24]. To further increa improved the reaction voltage platform and further enhanced the energy density of the electrofuclhl ebmattiecrayl[2c5h]a. NracFtePrOis4t(iNcFsP, N) has3Val2sOo 2axtt(rPacOte4d)2wFi3d-2ex,aattemntaiotenriinaslowdiutmh aioNn bAatSteICrieOs N stru for its electrochemical characteristics and higher theoretical capacity. It has three main similar to NVP, was reported by P. Ramesh Kumar and co-workers in Figure 5b. T structures, artificial crystalline salt type, olivine type and amorphous NaFePO4 [63]. herent electronegativity of F atoms present in these materials improved the reaction In order to obtain a relatively pure phase of olivine-type NaFePO4, Tang et al. used age platform and further enhanced the energy density of the full battery commercial LiFePO4, electrochemical delithium, and then intercalated sodium through aqueous solutions. Compared with the use of organic solutions to intercalate sodium, NaFePO4(NFP) has also attracted wide attention in sodium ion batteries for its el aqueous solutions are undoubtedly kinetically faster. By this method, higher purity is chemical characteristics and higher theoretical capacity. It has three main structures obtained, and the performance of NFP will be more stable. With 1 M Na2SO4, it can ficial crystalline salt type, olivine type and amo−r1phous NaFePO4 [63]. In order to ob reach a specific discharge capacity of 130 mAh g , and it has been cycled 1000 times, The capacity retention rate of the material can reach 80% [63–65]. Additionally, similar to the modification method of NVP, NFP can also slightly increase the voltage platform of the material on the basis of the original material by introducing F. Lalit Sharm et al. prepared Na2FePO4F as cathode, and designed a full battery composed of NaTi2(PO4)3 for anode material which can achieve a reversible specific capacity of 90 mAh g−1 in 17 M NaClO4 aqueous electrolyte, as shown in Figure 5c. In addition, the researchers proved that the battery could still achieve a high level of performance when the voltage is increased [52]. r a d s a n a r s h e tPDF Image | Aqueous Rechargeable Sodium-Ion Batteries Hydrogel
PDF Search Title:
Aqueous Rechargeable Sodium-Ion Batteries HydrogelOriginal File Name Searched:
batteries-08-00180-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |