
PDF Publication Title:
Text from PDF Page: 009
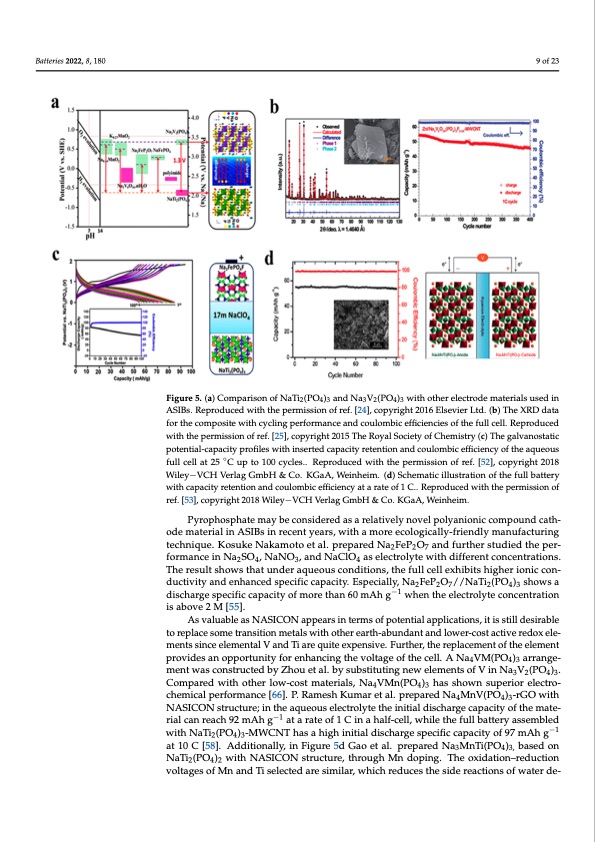
mance of NFP will be more stable. With 1 M Na2SO4, it can reach a specific discharge capacity of 130 mAh g−1, and it has been cycled 1000 times, The capacity retention rate of the material can reach 80% [63–65]. Batteries 2022, 8, 180 9 of 23 Figure 5. (a) Comparison of NaTi2(PO4)3 and Na3V2(PO4)3 with other electrode materials used in ASIBs. Reproduced with the permission of ref. [24], copyright 2016 Elsevier Ltd. (b) The XRD data Figure 5. (a) Comparison of NaTi2(PO4)3 and Na3V2(PO4)3 with other electrode materials used in for the composite with cycling performance and coulombic efficiencies of the full cell. Reproduced ASIBs. Reproduced with the permission of ref. [24], copyright 2016 Elsevier Ltd. (b) The XRD data with the permission of ref. [25], copyright 2015 The Royal Society of Chemistry (c) The galvanostatic for the composite with cycling performance and coulombic efficiencies of the full cell. Reproduced potential-capacity profiles with inserted capacity retention and coulombic efficiency of the aqueous with the permission of ref. [25], copyright 2015 The Royal Society of Chemistry (c) The galvanostatic full cell at 25 ◦C up to 100 cycles.. Reproduced with the permission of ref. [52], copyright 2018 potential-capacity profileWs wileiyt−hViCnHseVreterldagcGampabcHit&yCreo.teKnGtaioAn, Waenindhceoimu.lo(dm) Sbcihcemefafticiiellnusctyraotiofnthoef tahqe ufuellobuasttery with capacity retention and coulombic efficiency at a rate of 1 C.. Reproduced with the permission of full cell at 25°C up to 100 cycles.. Reproduced with the permission of ref. [49], copyright 2018 ref. [53], copyright 2018 Wiley−VCH Verlag GmbH & Co. KGaA, Weinheim. Wiley−VCH Verlag GmbH & Co. KGaA, Weinheim. (d) Schematic illustration of the full battery Pyrophosphate may be considered as a relatively novel polyanionic compound cath- with capacity retention and coulombic efficiency at a rate of 1 C.. Reproduced with the permission ode material in ASIBs in recent years, with a more ecologically-friendly manufacturing of ref. [50], copyright 2018 Wiley−VCH Verlag GmbH & Co. KGaA, Weinheim. technique. Kosuke Nakamoto et al. prepared Na2FeP2O7 and further studied the per- formance in Na2SO4, NaNO3, and NaClO4 as electrolyte with different concentrations. The result shows that under aqueous conditions, the full cell exhibits higher ionic con- Additionally, similar to the modification method of NVP, NFP can also slightly in- ductivity and enhanced specific capacity. Especially, Na2FeP2O7//NaTi2(PO4)3 shows a crease the voltage platform of the material on the basis of the original material by intro- discharge specific capacity of more than 60 mAh g−1 when the electrolyte concentration ducing F. Lalit Sharm eistaablo.vpe r2eMpa[5r5e]d. Na2FePO4F as cathode, and designed a full battery As valuable as NASICON appears in terms of potential applications, it is still desirable composed of NaTi2(PO4)3 for anode material which can achieve a reversible specific ca- to replace some transition metals with other earth-abundant and lower-cost active redox ele- pacity of 90 mAh g−1 in 17 M NaClO4 aqueous electrolyte, as shown in Figure 5c. In addi- ments since elemental V and Ti are quite expensive. Further, the replacement of the element tion, the researchers proved that the battery could still achieve a high level of performance provides an opportunity for enhancing the voltage of the cell. A Na4VM(PO4)3 arrange- ment was constructed by Zhou et al. by substituting new elements of V in Na V (PO ) . when the voltage is increased [49]. 3 2 4 3 Compared with other low-cost materials, Na4VMn(PO4)3 has shown superior electro- Pyrophosphate may be considered as a relatively novel polyanionic compound cath- chemical performance [66]. P. Ramesh Kumar et al. prepared Na4MnV(PO4)3-rGO with ode material in ASIBs in recent years, with a more ecologically-friendly manufacturing NASICON structure; in the aqueous electrolyte the initial discharge capacity of the mate- −1 rial can reach 92 mAh g at a rate of 1 C in a half-cell, while the full battery assembled technique. Kosuke Nakamoto et al. prepared Na2FeP2O7 and further studied the perfor- with NaTi (PO ) -MWCNT has a high initial discharge specific capacity of 97 mAh g−1 243 mance in Na2SO4, NaNO3, and NaClO4 as electrolyte with different concentrations. The at 10 C [58]. Additionally, in Figure 5d Gao et al. prepared Na3MnTi(PO4)3, based on result shows that under aqueous conditions, the full cell exhibits higher ionic conductivity NaTi2(PO4)2 with NASICON structure, through Mn doping. The oxidation–reduction andenhancedspecificvoclatapgaesciotfyM.nEasnpdeTciaselleyc,teNdar2eFseimP2iOlar7,/w/NhiachTrie2(dPuOce4s)t3heshsiodwerseacatiodniscofhwaragteerde- specific capacity of more than 60 mAh g−1 when the electrolyte concentration is above 2 M [52].PDF Image | Aqueous Rechargeable Sodium-Ion Batteries Hydrogel
PDF Search Title:
Aqueous Rechargeable Sodium-Ion Batteries HydrogelOriginal File Name Searched:
batteries-08-00180-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |