
PDF Publication Title:
Text from PDF Page: 005
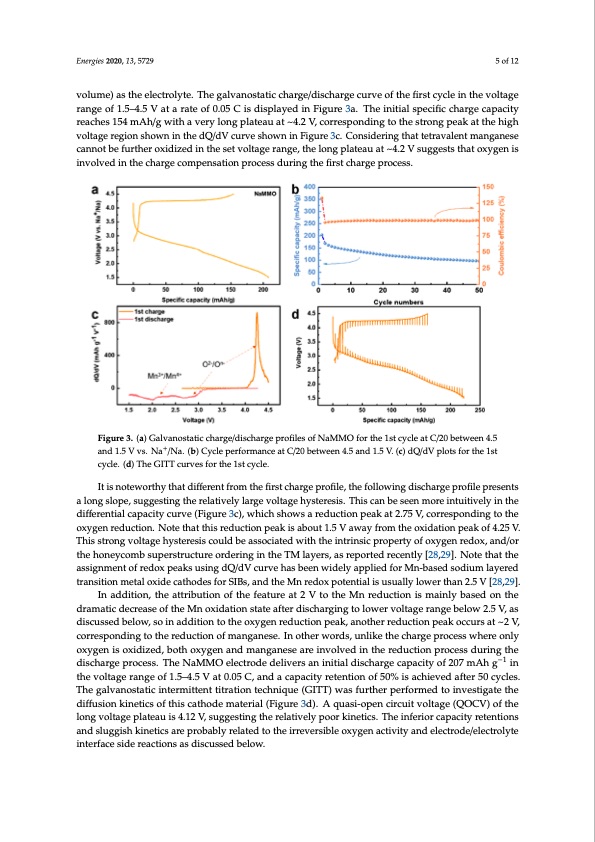
capacity reaches 154 mAh/g with a very long plateau at ~4.2 V, corresponding to the strong peak at the high voltage region shown in the dQ/dV curve shown in Figure 3c. Considering that tetravalent manganese cannot be further oxidized in the set voltage range, the long plateau at ~4.2 V suggests that oxygen is involved in the charge compensation process during the first charge process. It is noteworthy that different from the first charge profile, the following discharge profile Energies 2020, 13, 5729 5 of 12 presents a long slope, suggesting the relatively large voltage hysteresis. This can be seen more intuitively in the differential capacity curve (Figure 3c), which shows a reduction peak at 2.75 V, corresponding to the oxygen reduction. Note that this reduction peak is about 1.5 V away from the volume) as the electrolyte. The galvanostatic charge/discharge curve of the first cycle in the voltage oxidation peak of 4.25 V. This strong voltage hysteresis could be associated with the intrinsic property range of 1.5–4.5 V at a rate of 0.05 C is displayed in Figure 3a. The initial specific charge capacity of oxygen redox, and/or the honeycomb superstructure ordering in the TM layers, as reported reaches 154 mAh/g with a very long plateau at ~4.2 V, corresponding to the strong peak at the high recently [28,29]. Note that the assignment of redox peaks using dQ/dV curve has been widely applied voltage region shown in the dQ/dV curve shown in Figure 3c. Considering that tetravalent manganese for Mn-based sodium layered transition metal oxide cathodes for SIBs, and the Mn redox potential is cannot be further oxidized in the set voltage range, the long plateau at ~4.2 V suggests that oxygen is usually lower than 2.5 V [28,29]. involved in the charge compensation process during the first charge process. Figure 3. (a) Galvanostatic charge/discharge profifiles of NaMMO for the 1st cycle at C/20 between 4.5 + and 1.5 V vs. Na+//Na..(b(b))CCyyccleleppeerrffoorrmaancceeaattC//20bettween4..5and1..5V. (c) dQ/dV plots for the 1st cycle. (d) The GITT curves for the 1st cycle. It is noteworthy that different from the first charge profile, the following discharge profile presents In addition, the attribution of the feature at 2 V to the Mn reduction is mainly based on the a long slope, suggesting the relatively large voltage hysteresis. This can be seen more intuitively in the dramatic decrease of the Mn oxidation state after discharging to lower voltage range below 2.5 V, as differential capacity curve (Figure 3c), which shows a reduction peak at 2.75 V, corresponding to the discussed below, so in addition to the oxygen reduction peak, another reduction peak occurs at ~2 V, oxygen reduction. Note that this reduction peak is about 1.5 V away from the oxidation peak of 4.25 V. corresponding to the reduction of manganese. In other words, unlike the charge process where only This strong voltage hysteresis could be associated with the intrinsic property of oxygen redox, and/or oxygen is oxidized, both oxygen and manganese are involved in the reduction process during the thehoneycombsuperstructureorderingintheTMlayers,asreportedrecently[28,29].Notet-h1atthe discharge process. The NaMMO electrode delivers an initial discharge capacity of 207 mAh g in the assignment of redox peaks using dQ/dV curve has been widely applied for Mn-based sodium layered voltage range of 1.5–4.5 V at 0.05 C, and a capacity retention of 50% is achieved after 50 cycles. The transition metal oxide cathodes for SIBs, and the Mn redox potential is usually lower than 2.5 V [28,29]. galvanostatic intermittent titration technique (GITT) was further performed to investigate the In addition, the attribution of the feature at 2 V to the Mn reduction is mainly based on the diffusion kinetics of this cathode material (Figure 3d). A quasi-open circuit voltage (QOCV) of the dramatic decrease of the Mn oxidation state after discharging to lower voltage range below 2.5 V, as long voltage plateau is 4.12 V, suggesting the relatively poor kinetics. The inferior capacity retentions discussed below, so in addition to the oxygen reduction peak, another reduction peak occurs at ~2 V, and sluggish kinetics are probably related to the irreversible oxygen activity and electrode/electrolyte corresponding to the reduction of manganese. In other words, unlike the charge process where only interface side reactions as discussed below. oxygen is oxidized, both oxygen and manganese are involved in the reduction process during the discharge process. The NaMMO electrode delivers an initial discharge capacity of 207 mAh g−1 in the voltage range of 1.5–4.5 V at 0.05 C, and a capacity retention of 50% is achieved after 50 cycles. The galvanostatic intermittent titration technique (GITT) was further performed to investigate the diffusion kinetics of this cathode material (Figure 3d). A quasi-open circuit voltage (QOCV) of the long voltage plateau is 4.12 V, suggesting the relatively poor kinetics. The inferior capacity retentions and sluggish kinetics are probably related to the irreversible oxygen activity and electrode/electrolyte interface side reactions as discussed below.PDF Image | Cathode Materials for Advanced Sodium-Ion Batteries
PDF Search Title:
Cathode Materials for Advanced Sodium-Ion BatteriesOriginal File Name Searched:
charge-compensation-sodium-ion-battery.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |