
PDF Publication Title:
Text from PDF Page: 009
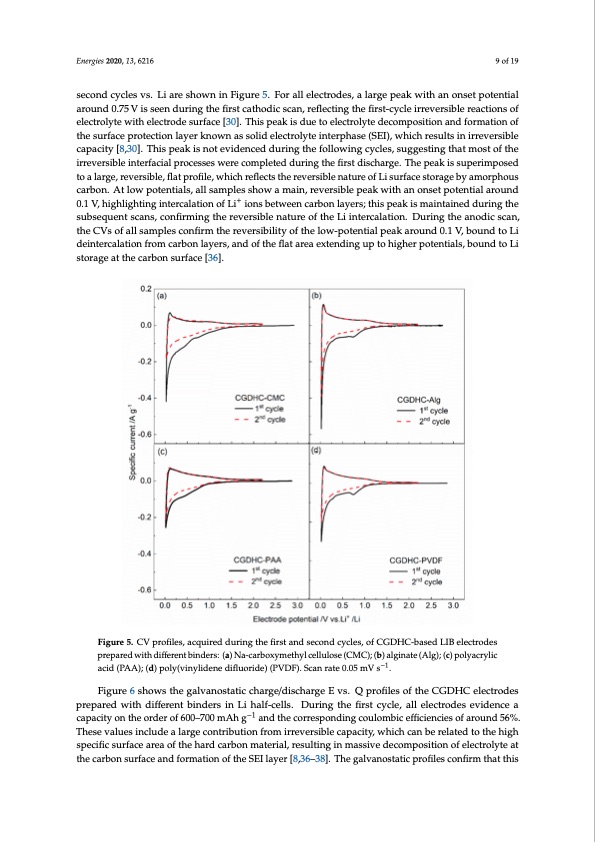
Energies 2020, 13, 6216 9 of 19 Esenecroginesd20c2y0c, l1e3s, xvFsO. RLPiEaErRe RsEhVoIwEWn in Figure 5. For all electrodes, a large peak with an onset pot9enofti2a0l around 0.75 V is seen during the first cathodic scan, reflecting the first-cycle irreversible reactions of reactions of electrolyte with electrode surface [30]. This peak is due to electrolyte decomposition and electrolyte with electrode surface [30]. This peak is due to electrolyte decomposition and formation of formation of the surface protection layer known as solid electrolyte interphase (SEI), which results in the surface protection layer known as solid electrolyte interphase (SEI), which results in irreversible irreversible capacity [8,30]. This peak is not evidenced during the following cycles, suggesting that capacity [8,30]. This peak is not evidenced during the following cycles, suggesting that most of the most of the irreversible interfacial processes were completed during the first discharge. The peak is irreversible interfacial processes were completed during the first discharge. The peak is superimposed superimposed to a large, reversible, flat profile, which reflects the reversible nature of Li surface to a large, reversible, flat profile, which reflects the reversible nature of Li surface storage by amorphous storage by amorphous carbon. At low potentials, all samples show a main, reversible peak with an carbon. At low potentials, all samples show a main, reversible peak with an onset potential around onset potential around 0.1 V, highlig+hting intercalation of Li+ ions between carbon layers; this peak 0.1 V, highlighting intercalation of Li ions between carbon layers; this peak is maintained during the is maintained during the subsequent scans, confirming the reversible nature of the Li intercalation. subsequent scans, confirming the reversible nature of the Li intercalation. During the anodic scan, During the anodic scan, the CVs of all samples confirm the reversibility of the low-potential peak the CVs of all samples confirm the reversibility of the low-potential peak around 0.1 V, bound to Li around 0.1 V, bound to Li deintercalation from carbon layers, and of the flat area extending up to deintercalation from carbon layers, and of the flat area extending up to higher potentials, bound to Li higher potentials, bound to Li storage at the carbon surface [36]. storage at the carbon surface [36]. Figure 5. CV profiles, acquired during the first and second cycles, of CGDHC-based LIB electrodes Figure 5. CV profiles, acquired during the first and second cycles, of CGDHC-based LIB electrodes prepared with different binders: (a) Na-carboxymethyl cellulose (CMC); (b) alginate (Alg); (c) polyacrylic prepared with different binders: (a) Na-carboxymethyl cellulose (CMC); (b) alginate (Alg); (c) acid (PAA); (d) poly(vinylidene difluoride) (PVDF). Scan rate 0.05 mV s−1. −1 polyacrylicacid(PAA);(d)poly(vinylidenedifluoride)(PVDF).Scanrate0.05mVs . Figure 6 shows the galvanostatic charge/discharge E vs. Q profiles of the CGDHC electrodes Figure 6 shows the galvanostatic charge/discharge E vs. Q profiles of the CGDHC electrodes prepared with different binders in Li half-cells. During the first cycle, all electrodes evidence a prepared with different binders in Li half-cells. During the first cycle, all electrodes evidence a capacity on the order of 600–700 mAh g−1 and the corresponding coulombic efficiencies of around 56%. capacity on the order of 600–700 mAh g−1 and the corresponding coulombic efficiencies of around These values include a large contribution from irreversible capacity, which can be related to the high 56%. These values include a large contribution from irreversible capacity, which can be related to the specific surface area of the hard carbon material, resulting in massive decomposition of electrolyte at high specific surface area of the hard carbon material, resulting in massive decomposition of the carbon surface and formation of the SEI layer [8,36–38]. The galvanostatic profiles confirm that this electrolyte at the carbon surface and formation of the SEI layer [8,36–38]. The galvanostatic profiles confirm that this process starts at around 0.80 V and carries on until about 0.20 V, where it is overlapped by a broad profile related to reversible Li+ ion intercalation and storage at the nanopore surface [36]. These results compare well with the features evidenced by the CV profiles.PDF Image | Coffee Ground Sustainable Anodes Sodium-Ion Batteries
PDF Search Title:
Coffee Ground Sustainable Anodes Sodium-Ion BatteriesOriginal File Name Searched:
energies-13-06216.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |