
PDF Publication Title:
Text from PDF Page: 010
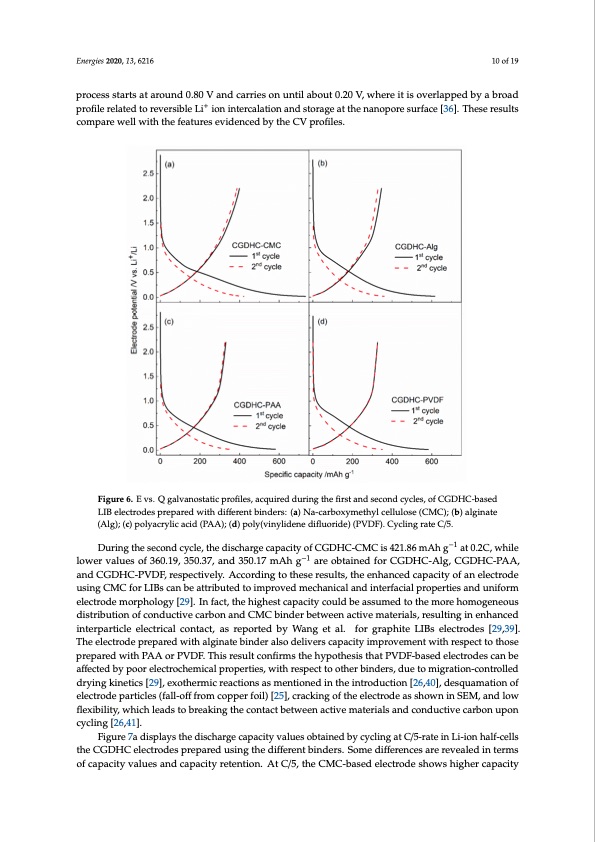
Energies 2020, 13, 6216 10 of 19 process starts at around 0.80 V and carries on until about 0.20 V, where it is overlapped by a broad profile related to reversible Li+ ion intercalation and storage at the nanopore surface [36]. These results compare well with the features evidenced by the CV profiles. Energies 2020, 13, x FOR PEER REVIEW 10 of 20 Figure 6..EEvvss..QQggaalvlvaannoosstatatitcicpprroofifileless,,aaccqquuirireeddurriingttheeffiirrssttaandsseeccondccycclless,,offCGDHC--based LIIB electrodes prepared with difffferent binders: (a) Na-carboxymetthyll celllullose (CMC));; (b) alginate (Alg); (c) polyacrylic acid (PAA); (d) poly(vinylidene diflfluoride) (PVDF). Cycling rate C/5.. −1 Duringthesecondcycle,thedischargecapacityofCGDHC-CMCis421.86mAhg–1 at0.2C,while During the second cycle, the discharge capacity of CGDHC-CMC is 421.86 mAh g at 0.2C, while lower values of 360.19, 350.37, and 350.17 mAh−1g−1 are obtained for CGDHC-Alg, CGDHC-PAA, lower values of 360.19, 350.37, and 350.17 mAh g are obtained for CGDHC-Alg, CGDHC-PAA, and and CGDHC-PVDF, respectively. According to these results, the enhanced capacity of an electrode CGDHC-PVDF, respectively. According to these results, the enhanced capacity of an electrode using using CMC for LIBs can be attributed to improved mechanical and interfacial properties and uniform CMC for LIBs can be attributed to improved mechanical and interfacial properties and uniform electrode morphology [29]. In fact, the highest capacity could be assumed to the more homogeneous electrode morphology [29]. In fact, the highest capacity could be assumed to the more homogeneous distribution of conductive carbon and CMC binder between active materials, resulting in enhanced distribution of conductive carbon and CMC binder between active materials, resulting in enhanced interparticle electrical contact, as reported by Wang et al. for graphite LIBs electrodes [29,39]. interparticle electrical contact, as reported by Wang et al. for graphite LIBs electrodes [29,39]. The The electrode prepared with alginate binder also delivers capacity improvement with respect to those electrode prepared with alginate binder also delivers capacity improvement with respect to those prepared with PAA or PVDF. This result confirms the hypothesis that PVDF-based electrodes can be prepared with PAA or PVDF. This result confirms the hypothesis that PVDF-based electrodes can be affected by poor electrochemical properties, with respect to other binders, due to migration-controlled affected by poor electrochemical properties, with respect to other binders, due to migration- drying kinetics [29], exothermic reactions as mentioned in the introduction [26,40], desquamation of controlled drying kinetics [29], exothermic reactions as mentioned in the introduction [26,40], electrode particles (fall-off from copper foil) [25], cracking of the electrode as shown in SEM, and low desquamation of electrode particles (fall-off from copper foil) [25], cracking of the electrode as shown flexibility, which leads to breaking the contact between active materials and conductive carbon upon in SEM, and low flexibility, which leads to breaking the contact between active materials and cycling [26,41]. conductive carbon upon cycling [26,41]. Figure 7a displays the discharge capacity values obtained by cycling at C/5-rate in Li-ion half-cells Figure 7a displays the discharge capacity values obtained by cycling at C/5-rate in Li-ion half- the CGDHC electrodes prepared using the different binders. Some differences are revealed in terms cells the CGDHC electrodes prepared using the different binders. Some differences are revealed in of capacity values and capacity retention. At C/5, the CMC-based electrode shows higher capacity terms of capacity values and capacity retention. At C/5, the CMC-based electrode shows higher capacity values through all over the investigated cycles in both conditions, because of a homogenous coating on the copper electrode. The capacity values for alginate- and PAA-based electrodes are initially lower, but they then exhibit better capacity retention, while the PVDF-based electrode shows slightly lower capacities. In order to further evaluate the effects of binders for LIBs, the capacity retention also were evaluated at 2C for 500 cycles as shown in Figure 7b, where the differences become even more marked, with the highest capacity values obtained by the CMC-based electrodePDF Image | Coffee Ground Sustainable Anodes Sodium-Ion Batteries
PDF Search Title:
Coffee Ground Sustainable Anodes Sodium-Ion BatteriesOriginal File Name Searched:
energies-13-06216.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |