
PDF Publication Title:
Text from PDF Page: 004
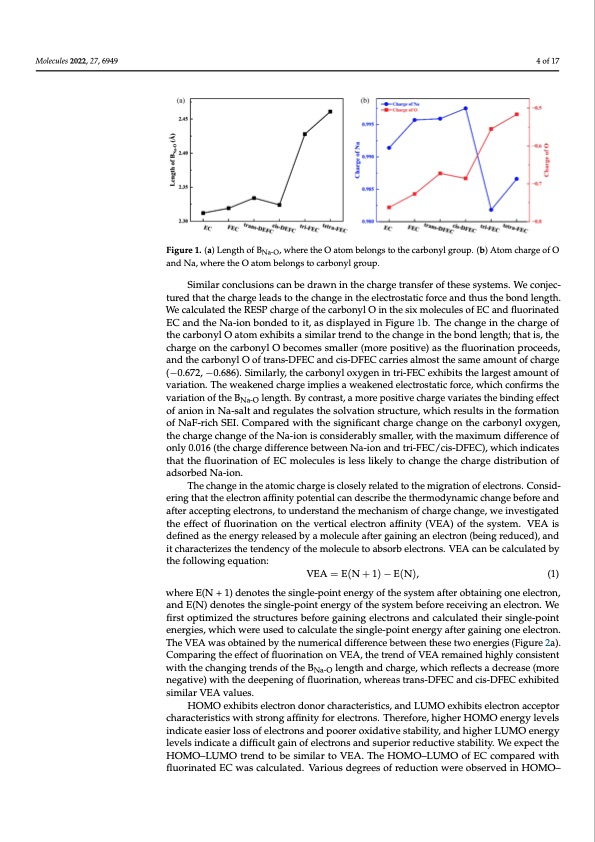
Molecules 2022, 27, x FOR PEER REVIEW 4 of 17 Molecules 2022, 27, 6949 4 of 17 Figure 1. (a) Length of BNa-O, where the O atom belongs to the carbonyl group. (b) Atom charge of Figure 1. (a) Length of BNa-O, where the O atom belongs to the carbonyl group. (b) Atom charge of O O and Na, where the O atom belongs to carbonyl group. and Na, where the O atom belongs to carbonyl group. Similar conclusions can be drawn in the charge transfer of these systems. We conjec- Similar conclusions can be drawn in the charge transfer of these systems. We conjec- tured that the charge leads to the change in the electrostatic force and thus the bond length. tured that the charge leads to the change in the electrostatic force and thus the bond length. We calculated the RESP charge of the carbonyl O in the six molecules of EC and fluorinated We calculated the RESP charge of the carbonyl O in the six molecules of EC and fluorinated EC and the Na-ionbondeedttooiti,t,aassddisipsplalyaeydedininFiFgiugruer1eb1.bT.hTehcehacnhgaengine tihnetchheacrhgaerogfethoef tchaerbcoanrbyolnOyaltOomateoxmhiebxithsiabistismailsairmtrileanrdtrteontdhetochthanegcehiantgheeibnotnhdelbeonngdthl;etnhgathis;,tthaetcish,atrhge cohnarthgeocanrbthoenyclarObobneycolmOebsescmomalelesrs(maolrlerp(omsiotirveep)oasitihve)flausotrhineafltiuonorpinraotcieoendsp,raoncedetdhse, acnadrbtohneyclaOrbofntyralnOs-oDfFtrEaCnsa-nDdFEciCs-DanFdECcisc-aDrFriEeCsaclamrroisetstahlemsoasmtethaemsaomunetaomfochuanrtgoef(c−h0a.6r7g2e, (−0.06.8667)2.,S−im0.i6la8r6ly).,Sthimecialarrbloyn,tyhleoxcyagrbeonninyltroix-FyEgCenexinhitbrit-sFtEhCeleaxrgheibstitasmthoeunlatrogfevsatraimatiounn.Ttohfe vwaeriaakteionne.dTchearwgeaikmepnleiedscahwaregaekiemnpedliesleactwroesatkaetincefdorecle,cwtrhoiscthatciconfofirrcme,swtheicvhacrioantifiornmosf the vBaNrai-Oatlieongothf.tBheyBcontraslet,nagmtho.rBeypcosnitirvaestc,haamrgoerveaprioastietsivtehechbainrgdeinvgaerifafetectsothfeanbiionndingNeaf-fseaclt Na-O oafnadnrieognuilnateNsat-hsealstoalvnadtiroengustlrautcetsutrhe,ewsohlivchatiroesnusltsruincttuhre,fowrmhiacthiornesoufltNsainF-trhicehfSoErmI.aCtoiomn- opfaNreadFw-riitchhthSeEsIi.gCniofmicapnatrcehdarwgeitchhtahnegesiogntifihecacnartbcohnayrlgoexcyhgaen,gteheonchtahregeccahrbanogneylofotxhyegNena-, the charge change of the Na-ion is considerably smaller, with the maximum difference of ion is considerably smaller, with the maximum difference of only 0.016 (the charge difference only 0.016 (the charge difference between Na-ion and tri-FEC/cis-DFEC), which indicates between Na-ion and tri-FEC/cis-DFEC), which indicates that the fluorination of EC molecules that the fluorination of EC molecules is less likely to change the charge distribution of is less likely to change the charge distribution of adsorbed Na-ion. adsorbed Na-ion. The change in the atomic charge is closely related to the migration of electrons. Consid- The change in the atomic charge is closely related to the migration of electrons. Consid- ering that the electron affinity potential can describe the thermodynamic change before and ering that the electron affinity potential can describe the thermodynamic change before and after accepting electrons, to understand the mechanism of charge change, we investigated the after accepting electrons, to understand the mechanism of charge change, we investigated effect of fluorination on the vertical electron affinity (VEA) of the system. VEA is defined as the effect of fluorination on the vertical electron affinity (VEA) of the system. VEA is the energy released by a molecule after gaining an electron (being reduced), and it charac- defined as the energy released by a molecule after gaining an electron (being reduced), and terizes the tendency of the molecule to absorb electrons. VEA can be calculated by the fol- it characterizes the tendency of the molecule to absorb electrons. VEA can be calculated by lowing equation: the following equation: VEA = E(N + 1) − E(N), (1) VEA = E(N+1)−E(N), (1) where E(N+1) denotes the single-point energy of the system after obtaining one electron, where E(N + 1) denotes the single-point energy of the system after obtaining one electron, and E(N) denotes the single-point energy of the system before receiving an electron. We first and E(N) denotes the single-point energy of the system before receiving an electron. We optimized the structures before gaining electrons and calculated their single-point energies, first optimized the structures before gaining electrons and calculated their single-point ewnheircghiews,ewrehuiscehdwtoercealucuseladtetothceaslcinuglalet-eptohientseingerleg-ypaofitnetr egnaeinrignygaofnteregleacitnriong. Tohne VelEeActrwoans. TohbetaVinEeAd bwyatshoebntauimnedricbayl dthifefenruenmcerbiceatlwdeieffnerthenesce tbwetoweenenrgtiheess(eFtigwuoren2aer).gCieosm(Fpiagruinreg2tah)e. Cefofemcpt oafrifnlugotrhineaetfifoenctoonfVflEuAor,itnhaettiorennodnoVf VEEAA, trheemtraeineddohfiVghElAy croenmsaisitneendt whiigthltyhecocnhsainsgteingt wtrietnhdtshoefcthaenBgNian-Ogletnregnthdsanodf tchearBge, whleicnhgrtehflaenctds achdaercgrea, sweh(michorreenflegcatstivaed)ewcritehasthee(mdeoerpe- Na-O neenginagtivoef)flwuoitrhintahteiodne,ewpheenrienagsotrfaflnus-oDriFnEaCtioan,dwchise-rDeFaEs Ctraenxsh-iDbiFteEdCsaimndilacrisV-DEAFEvCaleuxehsi.bited similaHrOVMEAOveaxlhuiebsi.ts electron donor characteristics, and LUMO exhibits electron acceptor charaHctOerMisOticesxwhitbhitstreolencgtraofnfindiotynfoorrcehleacrtarcotnesr.isTthicesr,eafonrde,LhUigMheOr eHxOhiMbiOtsenlecrgtryonlevaeclcseipntdoir- chaateraecatseireirstloicsswofitehlescttrongsanffidnpitoyofroereolxeicdtarotinves.sTtahbeirlietfyo,raen,dhihgihgehrerHLOUMOeenneerrgylleevells indicate aeadsiifefircluolstsgoaifneolefcetlreocntrsoannsdanpdoosurepreorixoirdraetdivuectsitvaebsiltiatby,ilaitnyd. WhiegehxepreLcUt tMheOHeOnMerOgy– levels indicate a difficult gain of electrons and superior reductive stability. We expect the LUMO trend to be similar to VEA. The HOMO–LUMO of EC compared with fluorinated EC HOMO–LUMO trend to be similar to VEA. The HOMO–LUMO of EC compared with was calculated. Various degrees of reduction were observed in HOMO–LUMO energy levels fluorinated EC was calculated. Various degrees of reduction were observed in HOMO– because of the fluorination in the Figure 2c. For solvent molecules, the reduced HOMO– LUMO energy level contributed to the decomposition of the anion, which generated anion-PDF Image | First-Principles-Based Optimized Design of Fluoride Electrolytes
PDF Search Title:
First-Principles-Based Optimized Design of Fluoride ElectrolytesOriginal File Name Searched:
molecules-27-06949.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |