
PDF Publication Title:
Text from PDF Page: 005
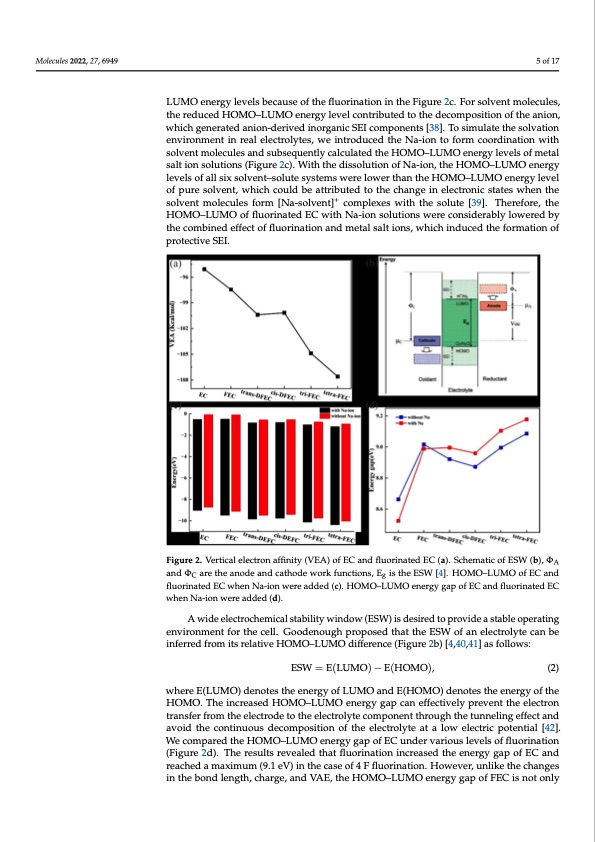
Molecules 2022, 27, 6949 5 of 17 Molecules 2022, 27, x FOR PEER REVIEW 5 of 17 LUMO energy levels because of the fluorination in the Figure 2c. For solvent molecules, the reduced HOMO–LUMO energy level contributed to the decomposition of the anion, which generated anion-derived inorganic SEI components [38]. To simulate the solvation derived inorganic SEI components [38]. To simulate the solvation environment in real elec- environment in real electrolytes, we introduced the Na-ion to form coordination with trolytes, we introduced the Na-ion to form coordination with solvent molecules and subse- solvent molecules and subsequently calculated the HOMO–LUMO energy levels of metal quently calculated the HOMO–LUMO energy levels of metal salt ion solutions (Figure 2c). salt ion solutions (Figure 2c). With the dissolution of Na-ion, the HOMO–LUMO energy With the dissolution of Na-ion, the HOMO–LUMO energy levels of all six solvent–solute levels of all six solvent–solute systems were lower than the HOMO–LUMO energy level systems were lower than the HOMO–LUMO energy level of pure solvent, which could be of pure solvent, which could be attributed to the change in electronic states when the attributed to the change in electronic states when the solvent molecules form [Na-solvent]+ solvent molecules form [Na-solvent]+ complexes with the solute [39]. Therefore, the complexes with the solute [39]. Therefore, the HOMO–LUMO of fluorinated EC with Na- HOMO–LUMO of fluorinated EC with Na-ion solutions were considerably lowered by ion solutions were considerably lowered by the combined effect of fluorination and metal the combined effect of fluorination and metal salt ions, which induced the formation of salt ions, which induced the formation of protective SEI. protective SEI. Figure 2. Vertical electron affinity (VEA) of EC and fluorinated EC (a). Schematic of ESW (b), ΦA and ΦC Figure 2. Vertical electron affinity (VEA) of EC and fluorinated EC (a). Schematic of ESW (b), ΦA are the anode and cathode work functions, Eg is the ESW [4]. HOMO–LUMO of EC and fluorinated EC and ΦC are the anode and cathode work functions, Eg is the ESW [4]. HOMO–LUMO of EC and when Na-ion were added (c). HOMO–LUMO energy gap of EC and fluorinated EC when Na-ion were fluorinated EC when Na-ion were added (c). HOMO–LUMO energy gap of EC and fluorinated EC added (d). when Na-ion were added (d). A wide electrochemical stability window (ESW) is desired to provide a stable oper- A wide electrochemical stability window (ESW) is desired to provide a stable operating ating environment for the cell. Goodenough proposed that the ESW of an electrolyte can environment for the cell. Goodenough proposed that the ESW of an electrolyte can be be inferred from its relative HOMO–LUMO difference (Figure 2b) [4,40,41] as follows: inferred from its relative HOMO–LUMO difference (Figure 2b) [4,40,41] as follows: ESW = E(LUMO) − E(HOMO), (2) ESW = E(LUMO) − E(HOMO), (2) where E(LUMO) denotes the energy of LUMO and E(HOMO) denotes the energy of the where E(LUMO) denotes the energy of LUMO and E(HOMO) denotes the energy of the HOMO. The increased HOMO–LUMO energy gap can effectively prevent the electron trans- HOMO. The increased HOMO–LUMO energy gap can effectively prevent the electron fer from the electrode to the electrolyte component through the tunneling effect and avoid transfer from the electrode to the electrolyte component through the tunneling effect and the continuous decomposition of the electrolyte at a low electric potential [42]. We compared avoid the continuous decomposition of the electrolyte at a low electric potential [42]. the HOMO–LUMO energy gap of EC under various levels of fluorination (Figure 2d). The We compared the HOMO–LUMO energy gap of EC under various levels of fluorination results revealed that fluorination increased the energy gap of EC and reached a maximum (9.1 (Figure 2d). The results revealed that fluorination increased the energy gap of EC and eV) in the case of 4 F fluorination. However, unlike the changes in the bond length, charge, reached a maximum (9.1 eV) in the case of 4 F fluorination. However, unlike the changes and VAE, the HOMO–LUMO energy gap of FEC is not only considerably larger than that of in the bond length, charge, and VAE, the HOMO–LUMO energy gap of FEC is not only EC but is even larger than that of DFEC and tri-FEC and only slightly smaller than that of tetra-FEC. To reflect the real-world solution environment, we compared the energy gaps of the six systems in the presence of Na-ion (Figure 2d), which revealed that the presence ofPDF Image | First-Principles-Based Optimized Design of Fluoride Electrolytes
PDF Search Title:
First-Principles-Based Optimized Design of Fluoride ElectrolytesOriginal File Name Searched:
molecules-27-06949.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |