
PDF Publication Title:
Text from PDF Page: 007
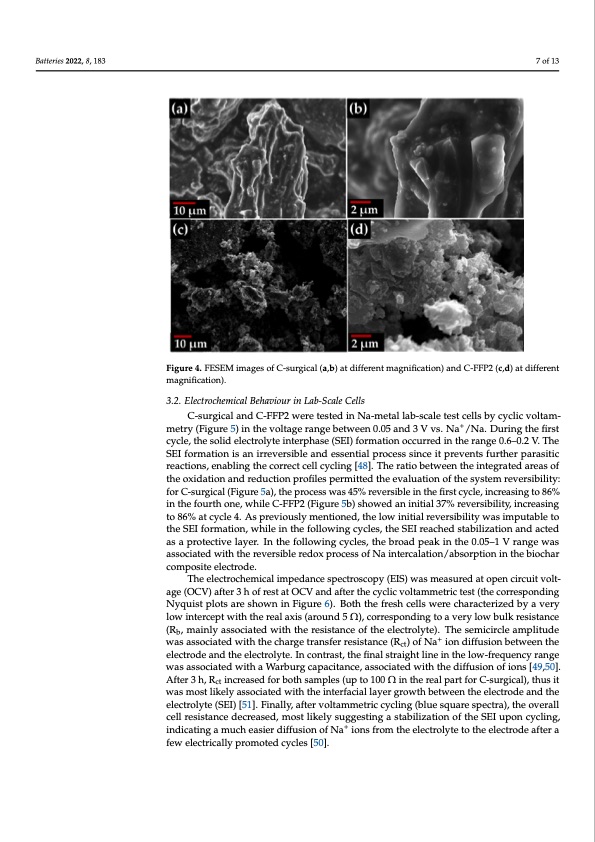
Batteries 2022, 8, 183 The morphology of the pyrolytic char produced from the surgical FMs (Figure 4a,b) was characterized by particles having sizes in the range of a hundred microns with few submicrometric carbon particles attached, similar to what was observed by Yousef et al. on other FM-derived carbon materials [47]. The particle size distribution of the FFP2 FMs was slightly different, with an average size lower than 1 μm. The different morphology was in good agreement with the specific surface area, which was 2.1 m2 g−1 in the case of C-surgical and 10.2 m2 g−1 in the case of C-FFP2, as measured by N2 physisorption analysis at −196 °C. 7 of 13 FFigiguurre4. FESEMimimagaegseosfoCf-Csu-rsguircgailc(al,b()aa,bt )diaftfedreifnftermeangtnmifiacgatnioifinc)atniodnC)-aFnFPd2C(c-,FdF)Pat2d(icf,fder)eanttdifferent magnification). magnification). 3.2. Electrochemical Behaviour in Lab-Scale Cells C-surgical and C-FFP2 were tested in Na-metal lab-scale test cells by cyclic voltam- metry (Figure 5) in the voltage range between 0.05 and 3 V vs. Na+/Na. During the first cycle, the solid electrolyte interphase (SEI) formation occurred in the range 0.6–0.2 V. The SEI formation is an irreversible and essential process since it prevents further parasitic reactions, enabling the correct cell cycling [48]. The ratio between the integrated areas of the oxidation and reduction profiles permitted the evaluation of the system reversibility: for C-surgical (Figure 5a), the process was 45% reversible in the first cycle, increasing to 86% in the fourth one, while C-FFP2 (Figure 5b) showed an initial 37% reversibility, increasing to 86% at cycle 4. As previously mentioned, the low initial reversibility was imputable to the SEI formation, while in the following cycles, the SEI reached stabilization and acted as a protective layer. In the following cycles, the broad peak in the 0.05–1 V range was associated with the reversible redox process of Na intercalation/absorption in the biochar composite electrode. The electrochemical impedance spectroscopy (EIS) was measured at open circuit volt- age (OCV) after 3 h of rest at OCV and after the cyclic voltammetric test (the corresponding Nyquist plots are shown in Figure 6). Both the fresh cells were characterized by a very low intercept with the real axis (around 5 Ω), corresponding to a very low bulk resistance (Rb, mainly associated with the resistance of the electrolyte). The semicircle amplitude was associated with the charge transfer resistance (Rct) of Na+ ion diffusion between the electrode and the electrolyte. In contrast, the final straight line in the low-frequency range was associated with a Warburg capacitance, associated with the diffusion of ions [49,50]. After 3 h, Rct increased for both samples (up to 100 Ω in the real part for C-surgical), thus it was most likely associated with the interfacial layer growth between the electrode and the electrolyte (SEI) [51]. Finally, after voltammetric cycling (blue square spectra), the overall cell resistance decreased, most likely suggesting a stabilization of the SEI upon cycling, indicating a much easier diffusion of Na+ ions from the electrolyte to the electrode after a few electrically promoted cycles [50].PDF Image | From Wastes to Anode Materials for Na-Ion Batteries
PDF Search Title:
From Wastes to Anode Materials for Na-Ion BatteriesOriginal File Name Searched:
batteries-08-00183.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |