
PDF Publication Title:
Text from PDF Page: 006
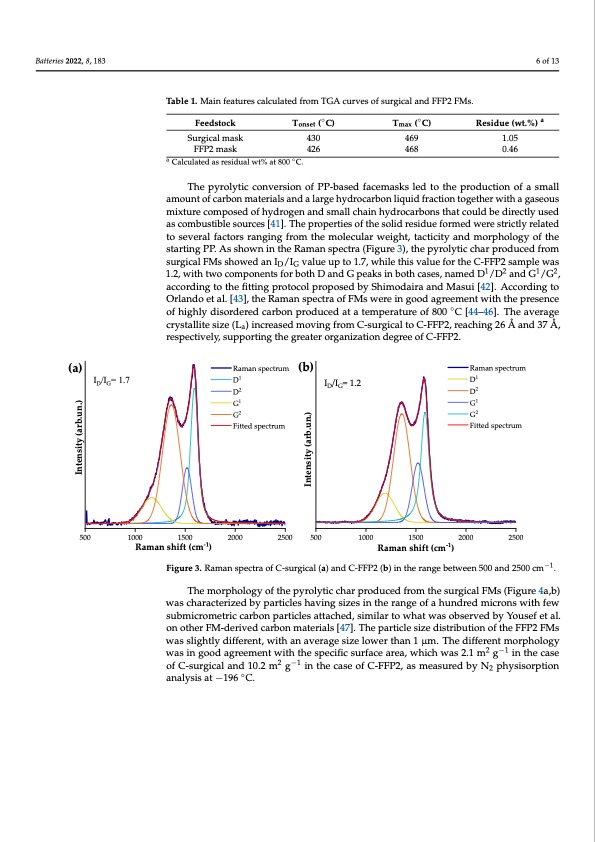
Batteries 2022, 8, 183 6 of 13 Table 1. Main features calculated from TGA curves of surgical and FFP2 FMs. Feedstock Surgical mask FFP2 mask Tonset (◦C) 430 426 Tmax (◦C) 469 468 Residue (wt.%) a 1.05 0.46 a Calculated as residual wt% at 800 ◦C. The pyrolytic conversion of PP-based facemasks led to the production of a small amount of carbon materials and a large hydrocarbon liquid fraction together with a gaseous mixture composed of hydrogen and small chain hydrocarbons that could be directly used as combustible sources [41]. The properties of the solid residue formed were strictly related to several factors ranging from the molecular weight, tacticity and morphology of the starting PP. As shown in the Raman spectra (Figure 3), the pyrolytic char produced from surgical FMs showed an ID/IG value up to 1.7, while this value for the C-FFP2 sample was 1.2, with two components for both D and G peaks in both cases, named D1/D2 and G1/G2, according to the fitting protocol proposed by Shimodaira and Masui [42]. According to Orlando et al. [43], the Raman spectra of FMs were in good agreement with the presence Batteries2022,8,xFORPEERREVIEW ofhighlydisorderedcarbonproducedatatemperatureof800◦C[44–46].Th7eofav15erage crystallite size (La) increased moving from C-surgical to C-FFP2, reaching 26 Å and 37 Å, respectively, supporting the greater organization degree of C-FFP2. (a) ID/IG= 1.7 Raman spectrum (b) D1 D2 G1 G2 Fitted spectrum 2000 2500 500 ID/IG= 1.2 Raman spectrum D1 D2 G1 G2 Fitted spectrum 2000 2500 Intensity (arb.un.) Intensity (arb.un.) 500 1000 1500 1000 1500 Raman shift (cm-1) Raman shift (cm-1) Figure3.RamanspectraofC-surgical(a)andC-FFP2(b)intherangebetween500and250−01 cm−1. Figure 3. Raman spectra of C-surgical (a) and C-FFP2 (b) in the range between 500 and 2500 cm . The morphology of the pyrolytic char produced from the surgical FMs (Figure 4a,b) The morphology of the pyrolytic char produced from the surgical FMs (Figure 4a,b) was characterized by particles having sizes in the range of a hundred microns with few was characterized by particles having sizes in the range of a hundred microns with few submicrometric carbon particles attached, similar to what was observed by Yousef et al. submicrometric carbon particles attached, similar to what was observed by Yousef et al. on on other FM-derived carbon materials [47]. The particle size distribution of the FFP2 FMs other FM-derived carbon materials [47]. The particle size distribution of the FFP2 FMs was was slightly different, with an average size lower than 1 μm. The different morphology slightly different, with an average size lower than 1 μm. The different morphology was in was in good agreement with the specific surface area, which was 2.1 m2 g−1 in the case good agreement with the specific surface area, which was 2.1 m2 g−1 in the case of C-surgical of C-surgical and 10.2 m2 g−1 in the case of C-FFP2, as measured by N2 physisorption and 10.2 m2 g−1 in the case of C-FFP2, as measured by N2 physisorption analysis at −196 °C. analysis at −196 ◦C.PDF Image | From Wastes to Anode Materials for Na-Ion Batteries
PDF Search Title:
From Wastes to Anode Materials for Na-Ion BatteriesOriginal File Name Searched:
batteries-08-00183.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |