
PDF Publication Title:
Text from PDF Page: 010
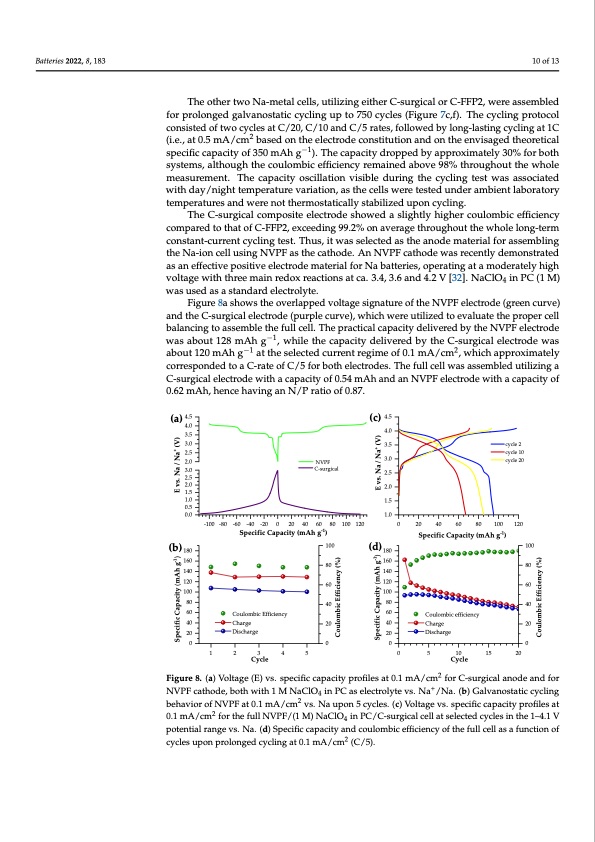
Batteries 2022, 8, x FOR PEER REVIEW 11 of 15 Batteries 2022, 8, 183 10 of 13 The other two Na-metal cells, utilizing either C-surgical or C-FFP2, were assembled The other two Na-metal cells, utilizing either C-surgical or C-FFP2, were assembled for prolonged galvanostatic cycling up to 750 cycles (Figure 7c,f). The cycling protocol for prolonged galvanostatic cycling up to 750 cycles (Figure 7c,f). The cycling protocol consisted of two cycles at C/20, C/10 and C/5 rates, followed by long-lasting cycling at 1C consisted of two cycles at C/20, C/10 and C/5 rates, followed by long-lasting cycling at 1C 22 (i.e., at 0.5 mA/cm based on the electrode constitution and on the envisaged theoretical (i.e.,at0.5mA/cm basedontheelectrodeconstitutionandontheenvisagedtheoretical −1−1 specific capacity of 350 mAh g ). The capacity dropped by approximately 30% for both specific capacity of 350 mAh g ). The capacity dropped by approximately 30% for both systems, although the coulombic efficiency remained above 98% throughout the whole systems, although the coulombic efficiency remained above 98% throughout the whole measurement. The capacity oscillation visible during the cycling test was associated with measurement. The capacity oscillation visible during the cycling test was associated day/night temperature variation, as the cells were tested under ambient laboratory with day/night temperature variation, as the cells were tested under ambient laboratory temperatures and were not thermostatically stabilized upon cycling. temperatures and were not thermostatically stabilized upon cycling. The C-surgical composite electrode showed a slightly higher coulombic efficiency The C-surgical composite electrode showed a slightly higher coulombic efficiency compared to that of C-FFP2, exceeding 99.2% on average throughout the whole long-term compared to that of C-FFP2, exceeding 99.2% on average throughout the whole long-term constant-current cycling test. Thus, it was selected as the anode material for assembling constant-current cycling test. Thus, it was selected as the anode material for assembling the Na-ion cell using NVPF as the cathode. An NVPF cathode was recently demonstrated the Na-ion cell using NVPF as the cathode. An NVPF cathode was recently demonstrated as an effective positive electrode material for Na batteries, operating at a moderately high as an effective positive electrode material for Na batteries, operating at a moderately high voltage with three main redox reactions at ca. 3.4, 3.6 and 4.2 V [32]. NaClO4 in PC (1 M) voltage with three main redox reactions at ca. 3.4, 3.6 and 4.2 V [32]. NaClO4 in PC (1 M) was used as a standard electrolyte. was used as a standard electrolyte. Figure 8a shows the overlapped voltage signature of the NVPF electrode (green Figure 8a shows the overlapped voltage signature of the NVPF electrode (green curve) curve) and the C-surgical electrode (purple curve), which were utilized to evaluate the and the C-surgical electrode (purple curve), which were utilized to evaluate the proper cell proper cell balancing to assemble the full cell. The practical capacity delivered by the balancing to assemble the full cell. The practical capacity delivered by the NVPF electrode −1 −1 NVPF electrode was about 128 mAh g , while the capacity delivered by the C-surgical was about 128 mAh g , while the capacity delivered by the C-surgical electrode was −1−1 22 electrode was about 120 mAh g at the selected current regime of 0.1 mA/cm , which about 120 mAh g at the selected current regime of 0.1 mA/cm , which approximately approximately corresponded to a C-rate of C/5 for both electrodes. The full cell was corresponded to a C-rate of C/5 for both electrodes. The full cell was assembled utilizing a assembled utilizing a C-surgical electrode with a capacity of 0.54 mAh and an NVPF C-surgical electrode with a capacity of 0.54 mAh and an NVPF electrode with a capacity of electrode with a capacity of 0.62 mAh, hence having an N/P ratio of 0.87. 0.62 mAh, hence having an N/P ratio of 0.87. (a) 4.5 4.0 3.5 3.0 2.5 2.0 3.0 2.5 2.0 1.5 1.0 0.5 0.0 (b) 180 160 140 120 100 80 60 40 20 Figure 8. (a) Voltage (E) vs. specific capacity profiles at 0.1 mA/c2m2 for C-surgical anode and for Figure 8. (a) Voltage (E) vs. specific capacity profiles at 0.1 mA/cm for C-surgical anode and for (c) 4.5 4.0 NVPF C-surgical 3.5 3.0 2.5 2.0 1.5 1.0 180 160 140 120 100 -100 -80 -60 -40 -20 0 20 40 60 80 100 120 0 20 40 60 80 100 120 Specific Capacity (mAh g-1) 100 (d) 80 60 Specific Capacity (mAh g-1) Coulombic Efficiency Charge Discharge 40 80 60 100 80 60 40 20 Coulombic efficiency 20 40 Charge 20 Discharge 0000 1 2 3 4 5 0 5 10 15 20 Cycle Cycle + NNVVPPFFccaaththoodde,e,bbooththwwitihth11MNaaCClOlO44ininPPCCaasseelelecctrtorolylytetevvs.s.NNaa/N/aN.a(b.)(bG)aGlvaalnvoasntoastitcatciycclyincgling + bbeehhaavvioiorrofoNfNVVPFPFata0t.10.m1Am/Acm/cmvs.Nvsa.uNpaounp5ocnyc5lecsy.c(lce)sV. o(clt)aVgoelvtasg.espvesc.ifsipceccaipfiacccitaypparcoitfyilepsraotfi0l.e1sat 22 mA/cm2 for 2the full NVPF/(1 M) NaClO4 in PC/C-surgical cell at selected cycles in the 1–4.1 V 0.1 mA/cm for the full NVPF/(1 M) NaClO4 in PC/C-surgical cell at selected cycles in the 1–4.1 V potential range vs. Na. (d) Specific capacity and coulombic efficiency of the full cell as a function of potential range vs. Na. (d) Specific capacity and coulombic efficiency of the full cell as a function of cycles upon prolonged cycling at 0.1 mA/cm2 (C/5). cycles upon prolonged cycling at 0.1 mA/cm2 (C/5). cycle 2 cycle 10 cycle 20 Specific Capacity (mAh g-1) E vs. Na / Na+ (V) Coulombic Efficiency (%) Specific Capacity (mAh g-1) E vs. Na / Na+ (V) Coulombic Efficiency (%)PDF Image | From Wastes to Anode Materials for Na-Ion Batteries
PDF Search Title:
From Wastes to Anode Materials for Na-Ion BatteriesOriginal File Name Searched:
batteries-08-00183.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |