
PDF Publication Title:
Text from PDF Page: 016
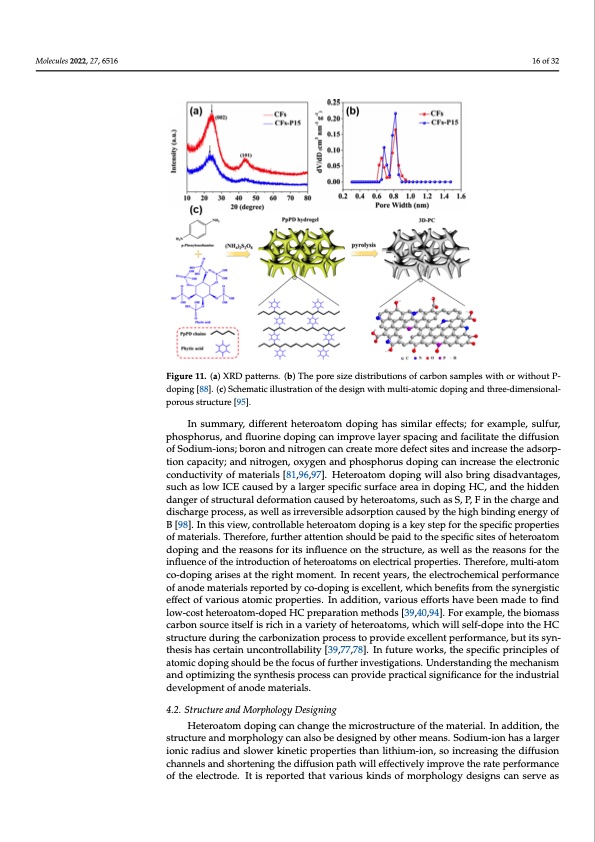
retention above 95% at 1000 cycles [94]. In contrast, Song’s Group [95] prepared Molecules 2022, 27, 6516 three-dimensional porous carbon materials with N, O and P co-doping by simple pyrol- ysis of the poly(P-phenylenediamine) (PpPD) hydrogel with phytic acid as a dopant and crosslinking agent. The mesoporous structures formed by the three-dimensional PpPD hydrogel network gives the anode material abundant defect sites and ion transport paths, and it serves a better electrochemical performance of 332 mAh g−1 at 0.05 A g−1, 139 mAh g−1 at 10 A g−1 and a wonderful cycling stability of 98.9% retention after 1000 cycles at 5 A g−1; this design is shown in Figure 11c. Figure 11. (a) XRD patterns. (b) The pore size distributions of carbon samples with or without Figure 11. (a) XRD patterns. (b) The pore size distributions of carbon samples with or without P- P-doping [88]. (c) Schematic illustration of the design with multi-atomic doping and doping [88]. (c) Schematic illustration of the design with multi-atomic doping and three-dimensional- porous structure [95]. In summary, different heteroatom doping has similar effects; for example, sulfur, phosphorus, and fluorine doping can improve layer spacing and facilitate the diffusion of phosphorus, and fluorine doping can improve layer spacing and facilitate the diffusion Sodium-ions; boron and nitrogen can create more defect sites and increase the adsorption of Sodium-ions; boron and nitrogen can create more defect sites and increase the adsorp- capacity; and nitrogen, oxygen and phosphorus doping can increase the electronic con- tion capacity; and nitrogen, oxygen and phosphorus doping can increase the electronic ductivity of materials [81,96,97]. Heteroatom doping will also bring disadvantages, such 16 of 32 three-dimensional-porous structure [95]. In summary, different heteroatom doping has similar effects; for example, sulfur, conductivity of materials [81,96,97]. Heteroatom doping will also bring disadvantages, as low ICE caused by a larger specific surface area in doping HC, and the hidden danger such as low ICE caused by a larger specific surface area in doping HC, and the hidden of structural deformation caused by heteroatoms, such as S, P, F in the charge and dis- danger of structural deformation caused by heteroatoms, such as S, P, F in the charge and charge process, as well as irreversible adsorption caused by the high binding energy of B discharge process, as well as irreversible adsorption caused by the high binding energy of [98]. In this view, controllable heteroatom doping is a key step for the specific properties B [98]. In this view, controllable heteroatom doping is a key step for the specific properties of materials. Therefore, further attention should be paid to the specific sites of heteroatom of materials. Therefore, further attention should be paid to the specific sites of heteroatom doping and the reasons for its influence on the structure, as well as the reasons for the doping and the reasons for its influence on the structure, as well as the reasons for the influence of the introduction of heteroatoms on electrical properties. Therefore, mul- influence of the introduction of heteroatoms on electrical properties. Therefore, multi-atom ti-atom co-doping arises at the right moment. In recent years, the electrochemical per- cfor-dmoapnicnegoafrainsoedseatmtahterriiaglshrtempormteednbt.yIcno-rdeocpenintgyiesaerxsc,etlhlenet,lewchtriochchbemneifciatslfpreormfotrhmeance osfynanerogdisetimcaetfeferciatlosfrevpaoriroteudsbatyomcoi-cdopproinpgeritsiees.xcIenllaedndt,itwiohni,chvabreionuesfitesfforrotmshthaveesybneenrgistic emffaedcet otof fvinardiolouws -actoosmt hicetperoaptoermti-edso.pIendaHdCdiptiroenp,avratrioionums etfhfordts [h3a9v,4e0,b94e]e.nFomraedxeamto- find lpolwe,-ctohsetbhieotmeraosastocmar-bdoonpesoduHrcCepitrseplfarisatrioicnhminethaovdasri[e3t9y,4o0f,9h4e].teFrorateoxmams,pwleh,icthewbiollmass carbon source itself is rich in a variety of heteroatoms, which will self-dope into the HC structure during the carbonization process to provide excellent performance, but its syn- thesis has certain uncontrollability [39,77,78]. In future works, the specific principles of atomic doping should be the focus of further investigations. Understanding the mechanism and optimizing the synthesis process can provide practical significance for the industrial development of anode materials. 4.2. Structure and Morphology Designing Heteroatom doping can change the microstructure of the material. In addition, the structure and morphology can also be designed by other means. Sodium-ion has a larger ionic radius and slower kinetic properties than lithium-ion, so increasing the diffusion channels and shortening the diffusion path will effectively improve the rate performance of the electrode. It is reported that various kinds of morphology designs can serve asPDF Image | Hard Carbons as Anodes in Sodium-Ion Batteries
PDF Search Title:
Hard Carbons as Anodes in Sodium-Ion BatteriesOriginal File Name Searched:
molecules-27-06516-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |