
PDF Publication Title:
Text from PDF Page: 020
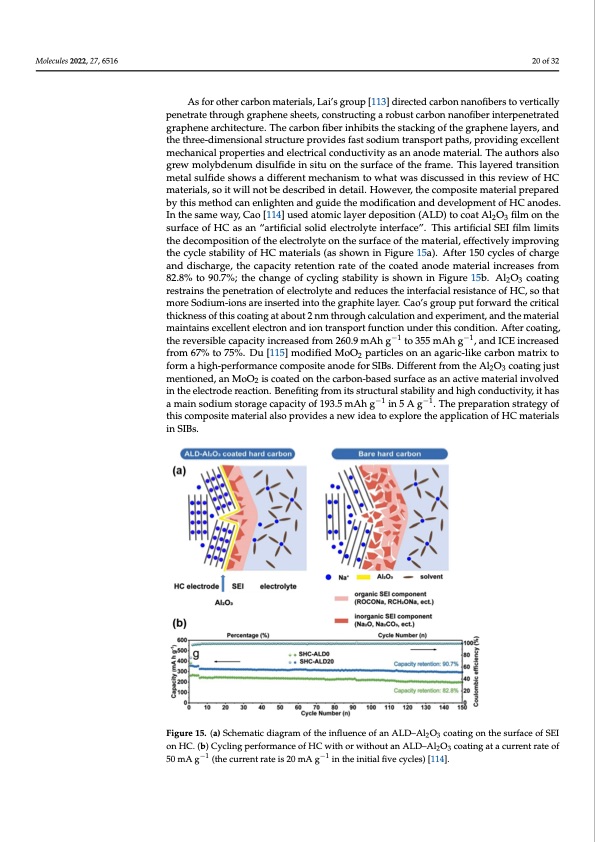
Molecules 2022, 27, 6516 20 of 32 As for other carbon materials, Lai’s group [113] directed carbon nanofibers to vertically penetrate through graphene sheets, constructing a robust carbon nanofiber interpenetrated graphene architecture. The carbon fiber inhibits the stacking of the graphene layers, and the three-dimensional structure provides fast sodium transport paths, providing excellent mechanical properties and electrical conductivity as an anode material. The authors also grew molybdenum disulfide in situ on the surface of the frame. This layered transition metal sulfide shows a different mechanism to what was discussed in this review of HC materials, so it will not be described in detail. However, the composite material prepared by this method can enlighten and guide the modification and development of HC anodes. In the same way, Cao [114] used atomic layer deposition (ALD) to coat Al2O3 film on the surface of HC as an “artificial solid electrolyte interface”. This artificial SEI film limits the decomposition of the electrolyte on the surface of the material, effectively improving the cycle stability of HC materials (as shown in Figure 15a). After 150 cycles of charge and discharge, the capacity retention rate of the coated anode material increases from 82.8% to 90.7%; the change of cycling stability is shown in Figure 15b. Al2O3 coating Molecules 2022, 27, x FOR PEER REVIErWestrains the penetration of electrolyte and reduces the interfacial resistance o2f2 HofC3,4so that more Sodium-ions are inserted into the graphite layer. Cao’s group put forward the critical thickness of this coating at about 2 nm through calculation and experiment, and the material maintains excellent electron and ion transport function under this condition. After coating, periment, and the material maintains excellent electron and ion transport function under the reversible capacity increased from 260.9 mAh g−1 to 355 mAh g−1, and −I1CE increased this condition. After coating, the reversible capacity increased from 260.9 mAh g to 355 from −617% to 75%. Du [115] modified MoO particles on an agaric-like carbon matrix to mAh g , and ICE increased from 67% to 75%. 2Du [115] modified MoO2 particles on an formahigh-performancecompositeanodeforSIBs.DifferentfromtheAlO coatingjust agaric-like carbon matrix to form a high-performance composite anode for SIBs.2Di3fferent frmomentthioenAeld2O,a3ncoMatoinOg2juistcmoaetnetdioonnedth,aencaMrbooOn2-ibsacsoeadtesduorfnatcheeacsaarbnoanc-tbiavseedmsauterfraiaceliansvolved aninatchteivelemctartoedriealreiancvtoiolvne.dBeinethfietinelgecfrtroomdeitrsesatcrtuioctnu.rBaelnsteafibtinligtyfaronmdhitisghstcrounctduuracltisvtiaty-,ithas −1 −1 bialimtyaaindsohdigihumconstdourcatgiveictya,piatchiatysaofm1a9i3n.5somdAiuhmgstoriange5cAapgacit.yTohfe1p93r.e5pmarAathiognsitnra5tegyof −1 Athgis.cTomhepporseitpeamraatitoenriastlraltseogyprofvtihdiesscaomnepwosiidteamtoaterxipaloarlesothperaopvpidliecsataionewof iHdeCamtoaterials −1 exinplSoIrBest.he application of HC materials in SIBs. Figure 15. (a) Schematic diagram of the influence of an ALD–Al2O3 coating on the surface of SEI on Figure 15. (a) Schematic diagram of the influence of an ALD–Al2O3 coating on the surface of SEI HC. (b) Cycling performance of HC with or without an ALD–Al2O3 coating at a current rate of 50 on HC. (b) Cycling performance of HC with or without an ALD–Al2O3 coating at a current rate of mA g−1 (the current rate is 20 mA g−1 in the initial five cycles) [114]. 50 mA g−1 (the current rate is 20 mA g−1 in the initial five cycles) [114]. As for the transition metal sulfides and oxides mentioned above, this conver- sion-type sodium storage mechanism shows a high sodium storage capacity, but it is limited by a low conductivity and volume change during the reaction process. The HC materials reviewed in this paper have a different intercalated sodium storage mechanism. They also exhibit high electron and ion transport efficiencies and stable mechanical properties, so they can be used to compensate for the defects of the former. ComparedPDF Image | Hard Carbons as Anodes in Sodium-Ion Batteries
PDF Search Title:
Hard Carbons as Anodes in Sodium-Ion BatteriesOriginal File Name Searched:
molecules-27-06516-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |