
PDF Publication Title:
Text from PDF Page: 006
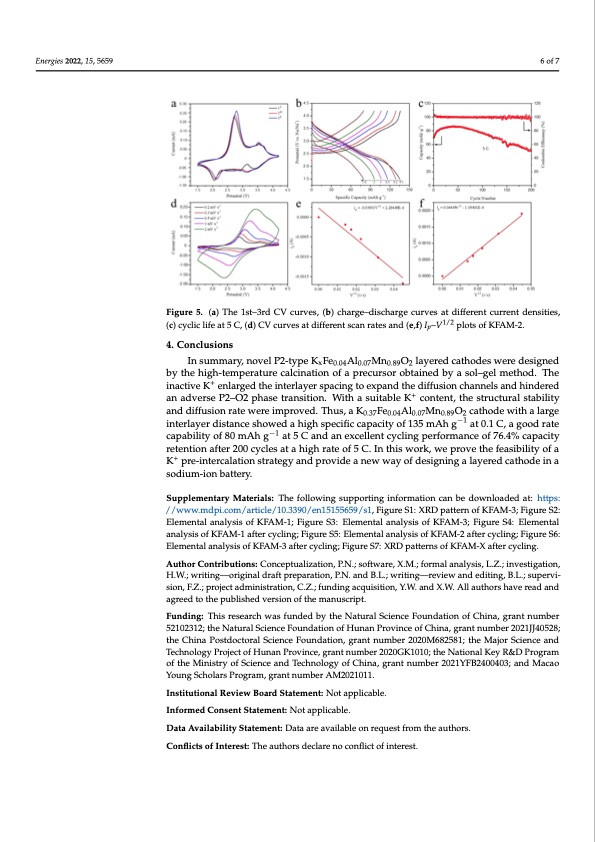
where I , n, A, D , C and V are the peak current, the number of electron transfers in the Energies 2022, 15, 5659 redox process, the contact area between the electrolyte and the active substance, the dif- fusion coefficient, the concentration of sodium ions in the lattice and the scan rate. It could be simplified to: Ip = 6487.6DNa1/2V1/2. Figure 5e,f were obtained according to the reduction and oxidation peaks at different scan rates. According the plots of Ip–V1/2, the diffusion coefficients were 3.68 × 10−11 and 4.69 × 10−11 cm2 s−1, respectively, reflecting the fast cha6rgofe7 transfer and storage of KFAM-2. Figure 5. (a) The 1st–3rd CV curves, (b) charge–discharge curves at different current densities, Figure 5. (a) The 1st–3rd CV curves, (b) charge–discharge curves at different current densities, (c) (c) cyclic life at 5 C, (d) CV curves at different scan rates and (e,f) I –V1/2 plots of KFAM-2. cyclic life at 5 C, (d) CV curves at different scan rates and (e,f) ip–Vp1/2 plots of KFAM-2. 4. Conclusions The XRD patterns of the KFAM-X after 50 cycles at 1 C were tested to detect their In summary, novel P2-type KxFe0.04Al0.07Mn0.89O2 layered cathodes were designed structural stability, as shown in Figure S7. There were no obvious changes for the charac- by the high-temperature calcination of a precursor obtained by a sol–gel method. The teristic peaks of KFAM-1 and KFAM-2, reflecting their good structural stability. A few inactive K+ enlarged the interlayer spacing to expand the diffusion channels and hindered peaks of NasFexAlyMnzO2 could be found in KFAM-2, revealing its poor structural stability an adverse P2–O2 phase transition. With a suitable K+ content, the structural stability and phase transformation. and diffusion rate were improved. Thus, a K0.37Fe0.04Al0.07Mn0.89O2 cathode with a large interlayer distance showed a high specific capacity of 135 mAh g−1 at 0.1 C, a good rate 4. Conclusions −1 capability of 80 mAh g at 5 C and an excellent cycling performance of 76.4% capacity In summary, novel P2-type KxFe0.04Al0.07Mn0.89O2 layered cathodes were designed by retention after 200 cycles at a high rate of 5 C. In this work, we prove the feasibility of a the high-temperature calcination of a precursor obtained by a sol–gel method. The inac- K+ pre-intercalation strategy and provide a new way of designing a layered cathode in a tive K+ enlarged the interlayer spacing to expand the diffusion channels and hindered an sodium-ion battery. adverse P2–O2 phase transition. With a suitable K+ content, the structural stability and dSiuffpupslieomnernattaerywMeraeteirmiaplsr:oTvheed.foTlhlouwsi,nagKsu0.p37pFoer0t.0i4nAgl0i.n07fMornm0a.8t9iOon2 catnhobededowitnhloadlaedrgaet:inhttetpr-s: −1 l/a/ywerwdwis.mtadnpcie.csohmo/waretdiclae/h1i0g.h33s9p0e/ceinf1ic51c5a5p6a5c9i/tys1o,Ffi1g3u5remS1A:hXRgDpatat0te.1rnCo,faKgFoAoMd-3r;aFteigcuarpeaS-2: −1 bEileitmyeonft8a0l amnAalyhsgis oaftK5FCAaMn-d1;aFnigeuxrcelSl3e:ntEcleymcleintgalpaenrfaolyrmsisaonfceKoFAf 7M6.-43%; FcigaupraecSit4y: rEeltemnteinotnal analysis of KFAM-1 after cycling; Figure S5: Elemental analysis of KFAM-2 after cycling; Fig+ure S6: after 200 cycles at a high rate of 5 C. In this work, we prove the feasibility of a K pre- Elemental analysis of KFAM-3 after cycling; Figure S7: XRD patterns of KFAM-X after cycling. intercalation strategy and provide a new way of designing a layered cathode in a sodium- ion battery. Author Contributions: Conceptualization, P.N.; software, X.M.; formal analysis, L.Z.; investigation, H.W.; writing—original draft preparation, P.N. and B.L.; writing—review and editing, B.L.; supervi- Supplementary Materials: The following supporting information can be downloaded at: sion, F.Z.; project administration, C.Z.; funding acquisition, Y.W. and X.W. All authors have read and www.mdpi.com/xxx/s1, Figure S1: XRD pattern of KFAM-3; Figure S2: Elemental analysis of agreed to the published version of the manuscript. KFAM-1; Figure S3: Elemental analysis of KFAM-3; Figure S4: Elemental analysis of KFAM-1 after Funding: This research was funded by the Natural Science Foundation of China, grant number cycling; Figure S5: Elemental analysis of KFAM-2 after cycling; Figure S6: Elemental analysis of 52102312; the Natural Science Foundation of Hunan Province of China, grant number 2021JJ40528; KFAM-3 after cycling; Figure S7: XRD patterns of KFAM-X after cycling. the China Postdoctoral Science Foundation, grant number 2020M682581; the Major Science and Technology Project of Hunan Province, grant number 2020GK1010; the National Key R&D Program of the Ministry of Science and Technology of China, grant number 2021YFB2400403; and Macao Young Scholars Program, grant number AM2021011. Institutional Review Board Statement: Not applicable. Informed Consent Statement: Not applicable. Data Availability Statement: Data are available on request from the authors. Conflicts of Interest: The authors declare no conflict of interest.PDF Image | Material as a High-Performance Cathode Sodium-Ion Batteries
PDF Search Title:
Material as a High-Performance Cathode Sodium-Ion BatteriesOriginal File Name Searched:
energies-15-05659.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |