
PDF Publication Title:
Text from PDF Page: 004
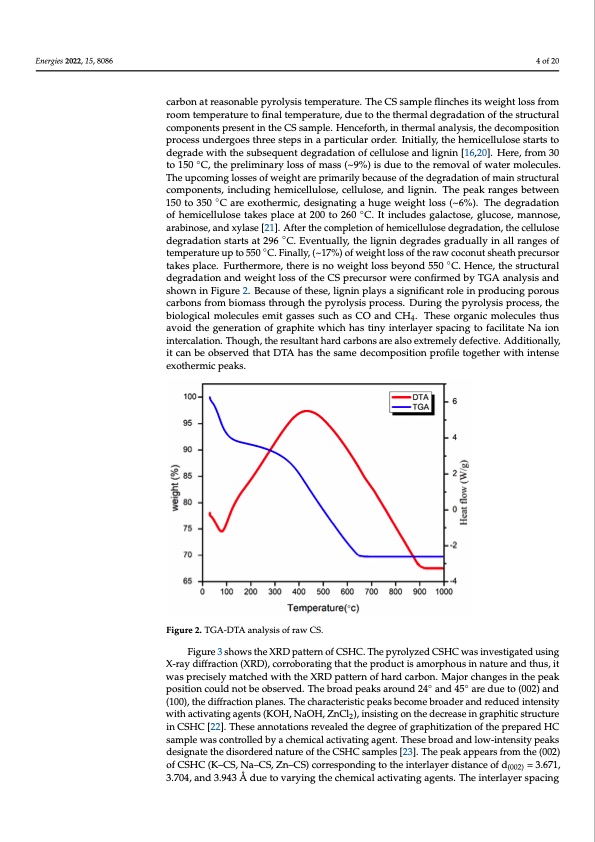
Energies 2022, 15, 8086 cludes high lignin content, hemicellulose, and cellulose. In this context, the hemicell and lignin are highly interconnected and amorphous, thus gratifying the emergen non-graphitic carbon at reasonable pyrolysis temperature. The CS sample flinche weight loss from room temperature to final temperature, due to the thermal degrad 4 of 20 of the structural components present in the CS sample. Henceforth, in thermal anal the decomposition process undergoes three steps in a particular order. Initially hemicellulose starts to degrade with the subsequent degradation of cellulose and li carbon at reasonable pyrolysis temperature. The CS sample flinches its weight loss from [16,20]. Here, from 30 to 150 °C, the preliminary loss of mass (~9%) is due to the rem room temperature to final temperature, due to the thermal degradation of the structural ocofmwpaotnerntms porlesceunlteisn.tThehCeSuspacmopmlei.nHgenlocsefsoerstho,finwtheeirgmhatlarnealpysrism, tahreildyecboemcpaoussiteioonf the d process undergoes three steps in a particular order. Initially, the hemicellulose starts to dation of main structural components, including hemicellulose, cellulose, and lignin. degrade with the subsequent degradation of cellulose and lignin [16,20]. Here, from 30 peak ranges between 150 to 350 °C are exothermic, designating a huge weight loss (~ to 150 ◦C, the preliminary loss of mass (~9%) is due to the removal of water molecules. The degradation of hemicellulose takes place at 200 to 260 °C. It includes galactose, The upcoming losses of weight are primarily because of the degradation of main structural cose, mannose, arabinose, and xylase [21]. After the completion of hemicellulose d components, including hemicellulose, cellulose, and lignin. The peak ranges between dation, the cellulose degradation starts at 296 °C. Eventually, the lignin degrades gr 150 to 350 ◦C are exothermic, designating a huge weight loss (~6%). The degradation ◦ aofllyheimnicaelllurlaonsgeetaskoefstpelmacpeearta2t0u0retou2p60toC5.5I0ti°nCcl.uFdiensagllayla,c(t~o1s7e,%g)luocfoswe,emigahntnlosses,ofthe arabinose, and xylase [21]. After the completion of hemicellulose degradation, the cellulose coconut sheath precursor takes place. Furthermore, there is no weight loss beyond degradation starts at 296 ◦C. Eventually, the lignin degrades gradually in all ranges of °C. Hence, the structural degradation and weight loss of the CS precursor were temperature up to 550 ◦C. Finally, (~17%) of weight loss of the raw coconut sheath precursor firmed by TGA analysis and shown in Figure 2. Because of these, lignin plays a si takes place. Furthermore, there is no weight loss beyond 550 ◦C. Hence, the structural cant role in producing porous carbons from biomass through the pyrolysis pro degradation and weight loss of the CS precursor were confirmed by TGA analysis and DshuorwinginthFiegupryer2o.lyBseicsaupsreoocfesthse,steh,eligbnioinlopglaicyaslamsioglneicfiucalenstreomleitingparsosdeuscsinugchpoarsouCsOand Tcahrebsoensofrgoamnibciomoalsesctuhrleosugthuthseapvyoriodlytshisepgreoncesrsa.tDiounrionfggthreaphyritoelywsishipcrhochesas,thineyinterl biological molecules emit gasses such as CO and CH . These organic molecules thus 4 spacing to facilitate Na ion intercalation. Though, the resultant hard carbons are als avoid the generation of graphite which has tiny interlayer spacing to facilitate Na ion tremely defective. Additionally, it can be observed that DTA has the same decompos intercalation. Though, the resultant hard carbons are also extremely defective. Additionally, profile together with intense exothermic peaks. it can be observed that DTA has the same decomposition profile together with intense exothermic peaks. Figure 2. TGA-DTA analysis of raw CS. Figure 2. TGA-DTA analysis of raw CS. Figure 3 shows the XRD pattern of CSHC. The pyrolyzed CSHC was investigated using X-rayFdigffurarcetio3ns(hXoRwD)s, ctohreroXboRrDatinpgathteartntheofprCodSuHcCt i.s TamheorphyoruoslyinzendatuCreSHanCd twhuass, iitnvestig was precisely matched with the XRD pattern of hard carbon. Major changes in the peak using X-ray diffraction (XRD), corroborating that the product is amorphous in nature position could not be observed. The broad peaks around 24◦ and 45◦ are due to (002) and thus, it was precisely matched with the XRD pattern of hard carbon. Major changes i (100), the diffraction planes. The characteristic peaks become broader and reduced intensity peak position could not be observed. The broad peaks around 24°and 45°are due to with activating agents (KOH, NaOH, ZnCl2), insisting on the decrease in graphitic structure and (100), the diffraction planes. The characteristic peaks become broader and red in CSHC [22]. These annotations revealed the degree of graphitization of the prepared HC isnamtepnlseiwtyaswcoitnhtroalcletidvbaytiancgheamgeicnatlsac(tKivOatHing, NagaeOntH. T,hZesneCbrlo2)a,dinansdisltoinwg-inotnentshitey pdeeackrsease in pdehsitgincastetrtuhcetduirseoridnerCedSHnaCtur[e22o]f.thTehCeSsHeCansnamotpalteiso[n23s].reTvheapleadktahpepedaersgfrreoemotfhegr(0a0p2h)itizati of CSHC (K–CS, Na–CS, Zn–CS) corresponding to the interlayer distance of d = 3.671, the prepared HC sample was controlled by a chemical activating agent. These broad 3.704, and 3.943 Å due to varying the chemical activating agents. The interlayer spacing low-intensity peaks designate the disordered nature of the CSHC samples [23]. The (002) u c a , g e e a g c C o i n ( u o pPDF Image | Morphology Derived Coconut Sheath for Sodium-Ion Battery
PDF Search Title:
Morphology Derived Coconut Sheath for Sodium-Ion BatteryOriginal File Name Searched:
energies-15-08086.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |