
PDF Publication Title:
Text from PDF Page: 003
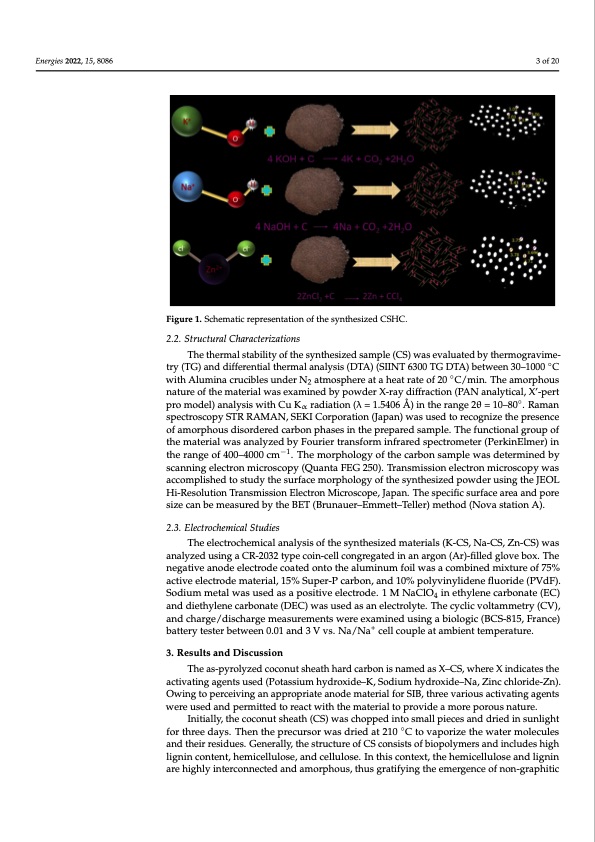
Energies 2022, 15, x FOR PEER REVIEW Energies 2022, 15, 8086 Figure 1. Schematic representation of the synthesized CSHC. Figure 1. Schematic representation of the synthesized CSHC. 2.2. Structural Characterizations The thermal stability of the synthesized sample (CS) was evaluated by therm The thermal stability of the synthesized sample (CS) was evaluated by thermogravime- try (TG) and differential thermal analysis (DTA) (SIINT 6300 TG DTA) between 30–1000 ◦C withAluminacruciblesunderN atmosphereataheatrateof20◦C/min.Theamorphous 2 vimetry (TG) and differential thermal analysis (DTA) (SIINT 6300 TG DTA) betwee nature of the material was examined by powder X-ray diffraction (PAN analytical, X’-pert 1000 °C with Alumina crucibles under N2 atmosphere at a heat rate of 20 °C/min pro model) analysis with Cu Kα radiation (λ = 1.5406 Å) in the range 2θ = 10–80◦. Raman amorphous nature of the material was examined by powder X-ray diffraction (PA 3 of 20 3 2.2. Structural Characterizations spectroscopy STR RAMAN, SEKI Corporation (Japan) was used to recognize the presence alytical, X’-pert pro model) analysis with Cu Kα radiation (λ = 1.5406 Å) in the rang of amorphous disordered carbon phases in the prepared sample. The functional group of th1e0–m8a0t°e.riRalawmasnansapleyczterdobscyoFpoyurSieTrRtraRnAsfMormANin,frSaEreKdIspCeocrtrpoomreateiron(Pe(JrakpinaEnlm)ewr)aisnusedt the range of 400–4000 cm−1. The morphology of the carbon sample was determined by ognize the presence of amorphous disordered carbon phases in the prepared sampl scanning electron microscopy (Quanta FEG 250). Transmission electron microscopy was functional group of the material was analyzed by Fourier transform infrared spectr accomplished to study the surface morphology of the synthesized powder using the JEOL ter (PerkinElmer) in the range of 400–4000 cm−1. The morphology of the carbon sa Hi-Resolution Transmission Electron Microscope, Japan. The specific surface area and pore was determined by scanning electron microscopy (Quanta FEG 250). Transmission size can be measured by the BET (Brunauer–Emmett–Teller) method (Nova station A). tron microscopy was accomplished to study the surface morphology of the synthe 2.3. Electrochemical Studies powder using the JEOL Hi-Resolution Transmission Electron Microscope, Japan The electrochemical analysis of the synthesized materials (K-CS, Na-CS, Zn-CS) was specific surface area and pore size can be measured by the BET (Brunauer–Em analyzed using a CR-2032 type coin-cell congregated in an argon (Ar)-filled glove box. The Teller) method (Nova station A). negative anode electrode coated onto the aluminum foil was a combined mixture of 75% active electrode material, 15% Super-P carbon, and 10% polyvinylidene fluoride (PVdF). 2.3. Electrochemical Studies Sodium metal was used as a positive electrode. 1 M NaClO4 in ethylene carbonate (EC) and diethylene carbonate (DEC) was used as an electrolyte. The cyclic voltammetry (CV), The electrochemical analysis of the synthesized materials (K-CS, Na-CS, Zn-CS and charge/discharge measurements were examined using a biologic (BCS-815, France) analyzed using a CR-2032 type coin-cell congregated in an argon (Ar)-filled glove battery tester between 0.01 and 3 V vs. Na/Na+ cell couple at ambient temperature. The negative anode electrode coated onto the aluminum foil was a combined mixt 75% active electrode material, 15% Super-P carbon, and 10% polyvinylidene flu 3. Results and Discussion (PVdF). Sodium metal was used as a positive electrode. 1 M NaClO4 in ethylen The as-pyrolyzed coconut sheath hard carbon is named as X–CS, where X indicates the abctoivnattineg(aEgCen)tsaunsdedd(Pieotahsysliuemnehycdarobxoidnea–tKe, S(DodEiuCm) hwydasroxuidse–dNa,sZainc cehlleocrtidroe-lZynte). The Owing to perceiving an appropriate anode material for SIB, three various activating agents voltammetry (CV), and charge/discharge measurements were examined using a bi were used and permitted to react with the material to provide a more porous+ nature. (BCS-815, France) battery tester between 0.01 and 3 V vs. Na/Na cell couple at am Initially, the coconut sheath (CS) was chopped into small pieces and dried in sunlight temperature. ◦ for three days. Then the precursor was dried at 210 C to vaporize the water molecules and their residues. Generally, the structure of CS consists of biopolymers and includes high 3. Results and Discussion lignin content, hemicellulose, and cellulose. In this context, the hemicellulose and lignin are highly interconnected and amorphous, thus gratifying the emergence of non-graphitic The as-pyrolyzed coconut sheath hard carbon is named as X–CS, where X ind the activating agents used (Potassium hydroxide–K, Sodium hydroxide–Na, Zinc ride-Zn). Owing to perceiving an appropriate anode material for SIB, three various vating agents were used and permitted to react with the material to provide a mor rous nature. Initially, the coconut sheath (CS) was chopped into small pieces and dried in o n N e o e . m ) u o e c o iPDF Image | Morphology Derived Coconut Sheath for Sodium-Ion Battery
PDF Search Title:
Morphology Derived Coconut Sheath for Sodium-Ion BatteryOriginal File Name Searched:
energies-15-08086.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |