
PDF Publication Title:
Text from PDF Page: 006
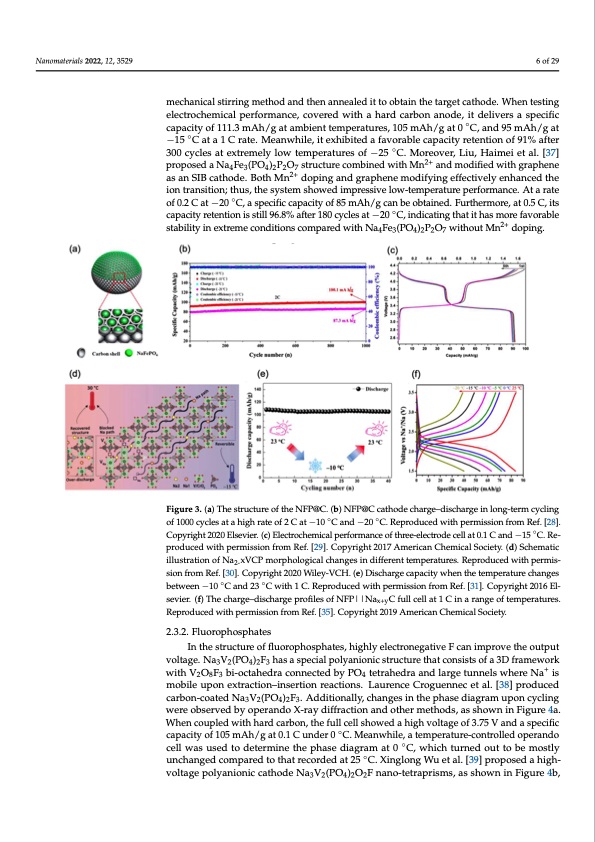
Na4Fe2(PO4)3 in cathode turned into Na5Fe2(PO4)3. Assembled with a hard carbon anode, the full cell delivered an ideal specific capacity of 74.6 mAh/g in 0 °C and 40 mAh/g at −20 °C with rate of 1 C, as shown in Figure 3f. Meanwhile, MNVP@C nano tubes were designed and synthesized by Changzhou Yuan et al. [36]. They first processed the raw Nanomaterials 2022, 12, 3529 material using a large-scale mechanical stirring method and then annealed it to obtain the target cathode. When testing electrochemical performance, covered with a hard car- bon anode, it delivers a specific capacity of 111.3 mAh/g at ambient temperatures, 105 6 of 29 mechanical stirring method and then annealed it to obtain the target cathode. When testing mAh/g at 0 °C, and 95 mAh/g at −15 °C at a 1 C rate. Meanwhile, it exhibited a favorable electrochemical performance, covered with a hard carbon anode, it delivers a specific capacity of 111.3 mAh/g at ambient temperatures, 105 mAh/g at 0 ◦C, and 95 mAh/g at capacity retention of 91% after 300 cycles at extremely low temperatures of −25 °C. More- ◦ over, Liu, Haimei et al. [37] proposed a Na4Fe3(PO4)2P2O7 structure combined with Mn −15 C at a 1 C rate. Meanwhile, it exhibited a favorable capacity retention of 91% after 2+ 300 cycles at extremely low temperatures of −25 ◦C. Moreover, Liu, Haimei et al. [37] and modified with graphene as an SIB cathode. Both Mn2+ doping and graphene modify- proposed a Na4Fe3(PO4)2P2O7 structure combined with Mn2+ and modified with graphene ing effectively enhanced the ion transition;2t+hus, the system showed impressive low- as an SIB cathode. Both Mn doping and graphene modifying effectively enhanced the temperatureperformionantrcaen.siAtiotna;trhautse,othfe0s.y2stCematsh−o2w0e°dCim,aprsepsseivceifliocwc-atepmapceirtaytuorfe8p5erfmorAmhan/gce.Atarate ◦ of 0.2 C at −20 C, a specific capacity of 85 mAh/g can be obtained. Furthermore, at 0.5 C, its can be obtained. Furthermore, at 0.5 C, its capacity retention is still 96.8% after 180 cycles capacity retention is still 96.8% after 180 cycles at −20 ◦C, indicating that it has more favorable at −20 °C, indicating that it has more favorable stability in extreme conditions compared stability in extreme conditions compared with Na Fe (PO ) P O without Mn2+ doping. with Na4Fe3(PO4)2P2O7 without Mn2+ doping. 434227 Figure 3. (a) The structure of the NFP@C. (b) NFP@C cathode charge–discharge in long-term cycling Figure 3. (a) The structure of the NFP@C. (b) NFP@C cathode charge–discharge in long-term cy- of 1000 cycles at a high rate of 2 C at −10 ◦C and −20 ◦C. Reproduced with permission from Ref. [28]. cling of 1000 cycles at a high rate of 2 C at −10 °C and −20 °C. Reproduced with permission from ◦ Copyright2020Elsevier.(c)Electrochemicalperformanceofthree-electrodecellat0.1Cand−15 C.Re- Ref. [28]. Copyright 2020 Elsevier. (c) Electrochemical performance of three-electrode cell at 0.1 C produced with permission from Ref. [29]. Copyright 2017 American Chemical Society. (d) Schematic and −15 °C. Reproduced with permission from Ref. [29]. Copyright 2017 American Chemical Soci- illustration of Na2-xVCP morphological changes in different temperatures. Reproduced with permis- ety. (d) Schematic illustration of Na2-xVCP morphological changes in different temperatures. Re- sion from Ref. [30]. Copyright 2020 Wiley-VCH. (e) Discharge capacity when the temperature changes produced with permission from R◦ ef. [30]. ◦Copyright 2020 Wiley-VCH. (e) Discharge capacity between −10 C and 23 C with 1 C. Reproduced with permission from Ref. [31]. Copyright 2016 El- when the temperature changes between −10 °C and 23 °C with 1 C. Reproduced with permission sevier. (f) The charge–discharge profiles of NFP||Nax+yC full cell at 1 C in a range of temperatures. from Ref. [31]. CopyriRgehptro2d0u1c6edEwlsiethviper.m(ifs)siTonhefrocmhaRregf.e[–3d5]i.sCcohpayrrgigehpt 2r0o1f9ilAems oerficNanFCPh|e|mNicaxl+SyoCcifeutyl.l cell at 1 C in a range of temperatures. Reproduced with permission from Ref. [35]. Copyright 2019 American Chemical Society. 2.3.2. Fluorophosphates In the structure of fluorophosphates, highly electronegative F can improve the output voltage.NaV(PO)F hasaspecialpolyanionicstructurethatconsistsofa3Dframework 2.3.2. Fluorophosphates 3 2 4 2 3 + with V2O8F3 bi-octahedra connected by PO4 tetrahedra and large tunnels where Na is mobile upon extraction–insertion reactions. Laurence Croguennec et al. [38] produced In the structure of fluorophosphates, highly electronegative F can improve the output voltage. Na3V2(PO4)2F3 has a special polyanionic structure that consists of a 3D framework carbon-coated Na3V2(PO4)2F3. Additionally, changes in the phase diagram upon cycling were observed by operando X-ray diffraction and other methods, as shown in Figure 4a. with V2O8F3 bi-octahedra connected by PO4 tetrahedra and large tunnels where Na+ is mo- When coupled with hard carbon, the full cell showed a high voltage of 3.75 V and a specific bile upon extraction–insertion reactions. Laurence Croguennec et al. [38] produced car- capacity of 105 mAh/g at 0.1 C under 0 ◦C. Meanwhile, a temperature-controlled operando ◦ bon-coated Na3V2(PcOel4l)2wFa3.sAusdeditoiodneatellrym,icnheatnhegepshainsetdhieagprhamaseatd0iagCr,awmhiuchptounrnceydcoliuntgtowberemostly unchanged compared to that recorded at 25 ◦C. Xinglong Wu et al. [39] proposed a high- voltage polyanionic cathode Na3V2(PO4)2O2F nano-tetraprisms, as shown in Figure 4b,PDF Image | Na Ion Batteries Used at Low Temperatures
PDF Search Title:
Na Ion Batteries Used at Low TemperaturesOriginal File Name Searched:
nanomaterials-12-03529-v4.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |