
PDF Publication Title:
Text from PDF Page: 007
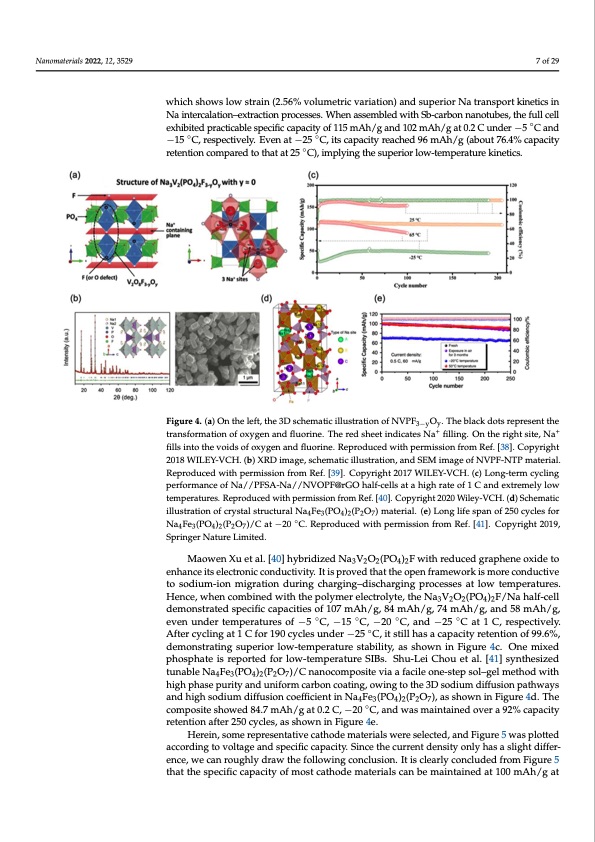
Nanomaterials 2022, 12, 3529 7 of 29 which shows low strain (2.56% volumetric variation) and superior Na transport kinetics in Na intercalation–extraction processes. When assembled with Sb-carbon nanotubes, the full cell exhibited practicable specific capacity of 115 mAh/g and 102 mAh/g at 0.2 C under −5 ◦C and −15 ◦C, respectively. Even at −25 ◦C, its capacity reached 96 mAh/g (about 76.4% capacity retention compared to that at 25 ◦C), implying the superior low-temperature kinetics. Figure 4. (a) On the left, the 3D schematic illustration of NVPF3−yOy. The black dots represent the transformation of oxygen and fluorine. The red sheet indicates Na+ filling. On the right site, Na+ fills into the voids of oxygen and fluorine. Reproduced with permission from Ref. [38]. Copyright 2018 WILEY-VCH. (b) XRD image, schematic illustration, and SEM image of NVPF-NTP material. Reproduced with permission from Ref. [39]. Copyright 2017 WILEY-VCH. (c) Long-term cycling performance of Na//PFSA-Na//NVOPF@rGO half-cells at a high rate of 1 C and extremely low temperatures. Reproduced with permission from Ref. [40]. Copyright 2020 Wiley-VCH. (d) Schematic illustration of crystal structural Na4Fe3(PO4)2(P2O7) material. (e) Long life span of 250 cycles for Na4Fe3(PO4)2(P2O7)/C at −20 ◦C. Reproduced with permission from Ref. [41]. Copyright 2019, Springer Nature Limited. Maowen Xu et al. [40] hybridized Na3V2O2(PO4)2F with reduced graphene oxide to enhance its electronic conductivity. It is proved that the open framework is more conductive to sodium-ion migration during charging–discharging processes at low temperatures. Hence, when combined with the polymer electrolyte, the Na3V2O2(PO4)2F/Na half-cell demonstrated specific capacities of 107 mAh/g, 84 mAh/g, 74 mAh/g, and 58 mAh/g, even under temperatures of −5 ◦C, −15 ◦C, −20 ◦C, and −25 ◦C at 1 C, respectively. After cycling at 1 C for 190 cycles under −25 ◦C, it still has a capacity retention of 99.6%, demonstrating superior low-temperature stability, as shown in Figure 4c. One mixed phosphate is reported for low-temperature SIBs. Shu-Lei Chou et al. [41] synthesized tunable Na4Fe3(PO4)2(P2O7)/C nanocomposite via a facile one-step sol–gel method with high phase purity and uniform carbon coating, owing to the 3D sodium diffusion pathways and high sodium diffusion coefficient in Na4Fe3(PO4)2(P2O7), as shown in Figure 4d. The composite showed 84.7 mAh/g at 0.2 C, −20 ◦C, and was maintained over a 92% capacity retention after 250 cycles, as shown in Figure 4e. Herein, some representative cathode materials were selected, and Figure 5 was plotted according to voltage and specific capacity. Since the current density only has a slight differ- ence, we can roughly draw the following conclusion. It is clearly concluded from Figure 5 that the specific capacity of most cathode materials can be maintained at 100 mAh/g atPDF Image | Na Ion Batteries Used at Low Temperatures
PDF Search Title:
Na Ion Batteries Used at Low TemperaturesOriginal File Name Searched:
nanomaterials-12-03529-v4.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |