
PDF Publication Title:
Text from PDF Page: 013
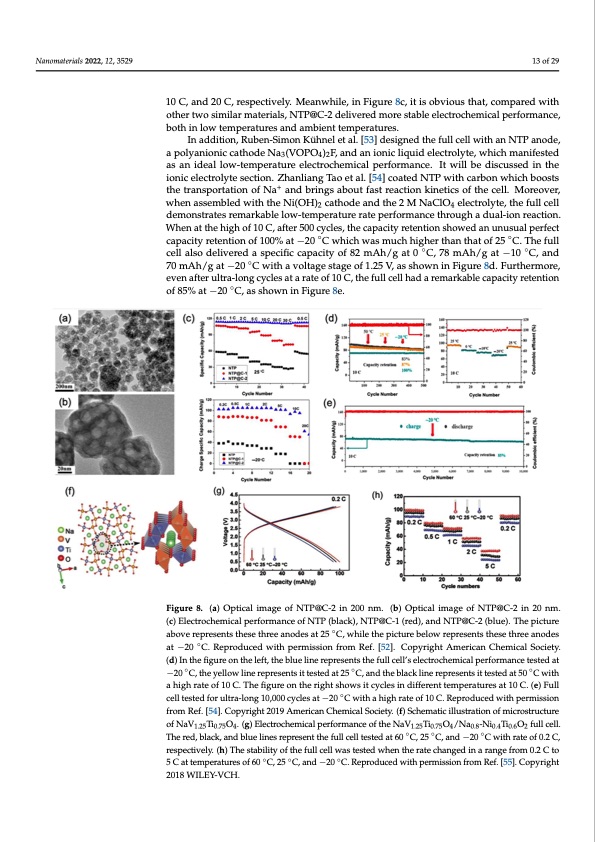
Nanomaterials 2022, 12, 3529 aterials 2022, 12, 3529 13 of 29 13 an idebaolthloinwlo-twemtemppeerraattures aenledcatmrobciehnetmtemicpaelrapteurefso.rmance. It will be discussed in the 10 C, and 20 C, respectively. Meanwhile, in Figure 8c, it is obvious that, compared with other two similar materials, NTP@C-2 delivered more stable electrochemical performance, In addition, Ruben-Simon Kühnel et al. [53] designed the full cell with an NTP anode, electrolyte section. Zhanliang Tao et al. [54] coated NTP with carbon which boos a polyanionic cathode Na (VOPO ) F, and an ionic liquid electrolyte, which manifested 342 transportation of Na+ and brings about fast reaction kinetics of the cell. Moreover, as an ideal low-temperature electrochemical performance. It will be discussed in the assembled with the Ni(OH)2 cathode and the 2 M NaClO4 electrolyte, the full cell de ionic electrolyte section. Zhanliang Tao et al. [54] coated NTP with carbon which boosts + stratesthremtraanrskpoarbtaletiolnowof-Nteampanedrabtruinrges raabtoeutpfearsftoreramctaionncekinthetriocsuogfhthae dceulla. lM-iorneorveear,ction. when assembled with the Ni(OH)2 cathode and the 2 M NaClO4 electrolyte, the full cell at the high of 10 C, after 500 cycles, the capacity retention showed an unusual p demonstrates remarkable low-temperature rate performance through a dual-ion reaction. capacity retention of 100% at −20 °C which was much higher than that of 25 °C. Th When at the high of 10 C, after 500 cycles, the capacity retention showed an unusual perfect cell also delivered a specific capacity of 82 mAh/g at 0 °C, 78 mAh/g at −10 °C, a capacity retention of 100% at −20 ◦C which was much higher than that of 25 ◦C. The full cell also delivered a specific capacity of 82 mAh/g at 0 ◦C, 78 mAh/g at −10 ◦C, and mAh/g at −20 °C with a voltage stage of 1.25 V, as shown in Figure 8d. Furthermore, 70 mAh/g at −20 ◦C with a voltage stage of 1.25 V, as shown in Figure 8d. Furthermore, after ultra-long cycles at a rate of 10 C, the full cell had a remarkable capacity retent even after ultra-long cycles at a rate of 10 C, the full cell had a remarkable capacity retention 85% at −20 °C, as s◦ hown in Figure 8e. of 85% at −20 C, as shown in Figure 8e. FigureF8ig.u(rae)8.O(pat)icOapltiicmalaimgeagoefoNf NTTPP@C-2iinn20200nmn.m(b.)(bO)ptOicpaltimcaalgeimofaNgeTPo@fCN-2TinP@20Cn-2m.in 20 n (c) Electrochemical performance of NTP (black), NTP@C-1 (red), and NTP@C-2 (blue). The picture Electrochemical performance of NTP (black), NTP@C-1 (red), and NTP@C-2 (blue). The p ◦ above represents these three anodes at 25 °C, while the picture below represents these three a above represents these three anodes at 25 C, while the picture below represents these three anodes at −20 ◦C. Reproduced with permission from Ref. [52]. Copyright American Chemical Society. at −20 °C. Reproduced with permission from Ref. [52]. Copyright American Chemical Society. (d) In the figure on the left, the blue line represents the full cell’s electrochemical performance tested at the figure on the left, the blue line represents the full cell’s electrochemical performance tes −20 ◦C, the yellow line represents it tested at 25 ◦C, and the black line represents it tested at 50 ◦C with −20 °C, the yellow line represents it tested at 25 °C, and the black line represents it tested at a high rate of 10 C. The figure on the right shows it cycles in different temperatures at 10 C. (e) Full with a high rate of 10 C. The figure on the◦right shows it cycles in different temperatures at 10 cell tested for ultra-long 10,000 cycles at −20 C with a high rate of 10 C. Reproduced with permission Full ceflrlotmesRtefd. [5fo4]r. Cuolptryari-glhotn2g01190A,0m0e0ricayncClehsemaitca−l2S0oc°ieCty.w(fi)tShchaemhaigtichilrluastteraotifon10ofCm.icRroesptrruoctdurueced wit missionof fNroaVm1.2R5Tei0f.7.5[O54.](.gC) Eolepcytrroicghhemt 2ic0al1p9erAfomrmearniceaonf tChehNemaVi1c.2a5lTiS0.o75cOie4/tyN.a(0f.8)-NSic0h.4Teim0.6Oat2icfuilllcuelsl.tration The red, black, and blue lines represent the full cell tested at 60 ◦C, 25 ◦C, and −20 ◦C with rate of 0.2 C, crostructure of NaV1.25Ti0.75O4. (g) Electrochemical performance of the NaV1.25Ti0.75O4 respectively. (h) The stability of the full cell was tested when the rate changed in a range from 0.2 C to Ni0.4Ti0.6O2 full cell. The red, black, and blue lines represent the full cell tested at 60 °C, 25 ° 5 C at temperatures of 60 ◦C, 25 ◦C, and −20 ◦C. Reproduced with permission from Ref. [55]. Copyright −20 °C with rate of 0.2 C, respectively. (h) The stability of the full cell was tested when th 2018 WILEY-VCH. changed in a range from 0.2 C to 5 C at temperatures of 60 °C, 25 °C, and −20 °C. Reproduce permission from Ref. [55]. Copyright 2018 WILEY-VCH. Besides the above-mentioned three types of anodes, high-crystallinity Ti-based m h / C d o NaV1.25Ti0.75O4 was also prepared and investigated in a high-crystalline structure as a t w W e n iPDF Image | Na Ion Batteries Used at Low Temperatures
PDF Search Title:
Na Ion Batteries Used at Low TemperaturesOriginal File Name Searched:
nanomaterials-12-03529-v4.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |