
PDF Publication Title:
Text from PDF Page: 006
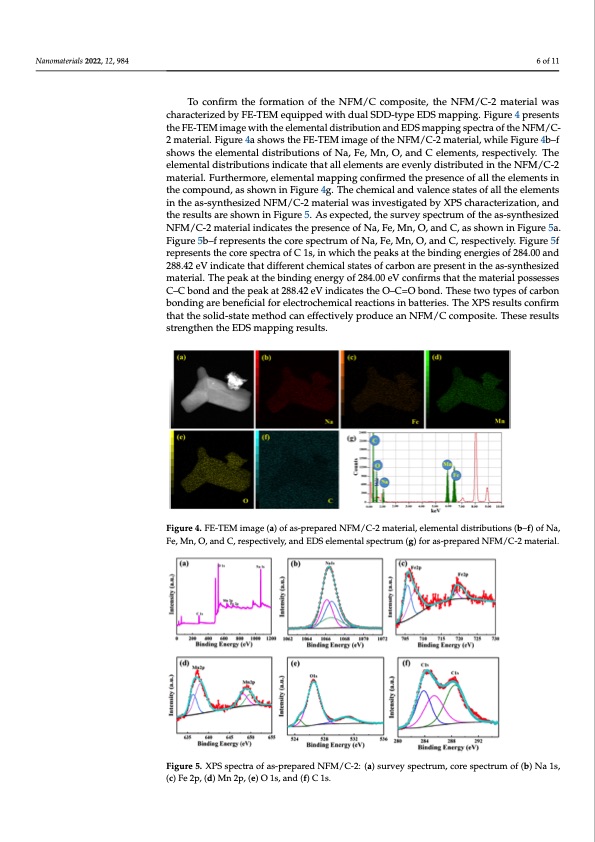
Nanomaterials 2022, 12, 984 6 of 11 Nanomaterials 2022, 12, 984 Nanomaterials 2022, 12, 984 material. Furthermore, elemental mapping confirmed the presence of all the elements in To confirm the formation of the NFM/C composite, the NFM/C-2 material was characterized by FE-TEM equipped with dual SDD-type EDS mapping. Figure 4 presents the FE-TEM image with the elemental distribution and EDS mapping spectra of the NFM/C- 2 material. Figure 4a shows the FE-TEM image of the NFM/C-2 material, while Figure 4b–f shows the elemental distributions of Na, Fe, Mn, O, and C elements, respectively. The elemental distributions indicate that all elements are evenly distributed in the NFM/C-2 7 of 12 the compound, as shown in Figure 4g. The chemical and valence states of 7alolf t1h2e elements in the as-synthesized NFM/C-2 material was investigated by XPS characterization, and the results are shown in Figure 5. As expected, the survey spectrum of the as-synthesized in the as-synthesized NFM/C-2 material was investigated by XPS characterization, and in the as-synthesized NFM/C-2 material was investigated by XPS characterization, and NFM/C-2 material indicates the presence of Na, Fe, Mn, O, and C, as shown in Figure 5a. the results are shown in Figure 5. As expected, the survey spectrum of the as-synthesized the results are shown in Figure 5. As expected, the survey spectrum of the as-synthesized FNigFuMre/C5-b2–mfartepriraelsienndtiscatthesetchoerpersepsenccteruomf Noaf,NFea,,MFne,OM,nan,dOC,a,nasdsCho,wrenspineFcitgivuerely5.aF.igure5f NFM/C-2 material indicates the presence of Na, Fe, Mn, O, and C, as shown in Figure 5a. reFpigruersen5bts–fthrepcroersenstpsethcteracoorfeCsp1esc,triunmwohficNhat,hFe,pMeank,sOa,tanthdeCb,irnedspinegctievneelyrg.Fieigsuorfe258f4.00and Figure 5b–f represents the core spectrum of Na, Fe, Mn, O, and C, respectively. Figure 5f represents the core spectra of C 1s, in which the peaks at the binding energies of 284.00 288.42 eV indicate that different chemical states of carbon are present in the as-synthesized represents the core spectra of C 1s, in which the peaks at the binding energies of 284.00 and 288.42 eV indicate that different chemical states of carbon are present in the as-syn- material. The peak at the binding energy of 284.00 eV confirms that the material possesses and 288.42 eV indicate that different chemical states of carbon are present in the as-syn- thesized material. The peak at the binding energy of 284.00 eV confirms that the material C–C bond and the peak at 288.42 eV indicates the O–C=O bond. These two types of carbon thesized material. The peak at the binding energy of 284.00 eV confirms that the material possesses C–C bond and the peak at 288.42 eV indicates the O–C=O bond. These two types bonding are beneficial for electrochemical reactions in batteries. The XPS results confirm possesses C–C bond and the peak at 288.42 eV indicates the O–C=O bond. These two types of carbon bonding are beneficial for electrochemical reactions in batteries. The XPS results tohfactartbhoensboolnid-instgaaterembentehfoicdialcfaonr elfefcetcroticvhelmyipcarlordeaucctieonasninNbFaMtte/riCes.cTohmepXoPsSitres.uTlthsese results confirm that the solid-state method can effectively produce an NFM/C composite. These sctornefnirgmthtehnattthe EsoDliSd-mstatpepminetghoredscualntse.ffectively produce an NFM/C composite. These results strengthen the EDS mapping results. results strengthen the EDS mapping results. FFigigurre 4. FE-TEMimimagaege(a()ao)foafs-apsr-eppraerpeadrNedFMN/FCM-2/mCa-t2ermiaal,telreimale,netlaelmdiesntrtiabludtiiosntrsi(bbu–tfi)oonfsN(ba,–f) of Na, Figure 4. FE-TEM image (a) of as-prepared NFM/C-2 material, elemental distributions (b–f) of Na, FFee,,Mn,,O, ,aanandndCC,Cr, e,rserpespescpetceitvcievtlieyvl,yea,lnya,dnadEnDEdDSESeDlelmSemenletnamtlaeslpnsetpcaetlrcustrpmuem(cgt)r(gufo)mrfoa(rsg-ap)sr-fepopreaapresad-rpeNrdeFNpMaF/rMCe-d/2CNm-2aFmtMeari/taeClr.i-a2l.material. Figure 5. XPS spectra of as-prepared NFM/C-2: (a) survey spectrum, core spectrum of (b) Na 1s, (c) FFigigurre5. XPSSspspecetcratroafoafs-apsr-epraerpedarNeFdMN/CF-M2:/(aC)-s2u:rv(ae)ysuprevcteryumsp, ceocrteruspme,ctcrourmeosfp(ebc)tNruam1so, (fc()b) Na 1s, Fe 2p, (d) Mn 2p, (e) O 1s, and (f) C 1s. Fe 2p, (d) Mn 2p, (e) O 1s, and (f) C 1s. (c) Fe 2p, (d) Mn 2p, (e) O 1s, and (f) C 1s. Figure 6 shows the dQ/dV curves of the as-prepared NFM, NFM/C-1, NFM/C-2, and Figure 6 shows the dQ/dV curves of the as-prepared NFM, NFM/C-1, NFM/C-2, and NFM/C-3 materials, respectively. The as-prepared materials have two redox peaks. The NFM/C-3 materials, respectively. The as-prepared materials have two redox peaks. The NFM material shows the oxidation peaks at 2.42 V and 3.92 V during the charge, and it NFM material shows the oxidation peaks at 2.42 V and 3.92 V during the charge, and itPDF Image | NaFe0 Nanocomposite as a Cathode for Sodium-Ion Batteries
PDF Search Title:
NaFe0 Nanocomposite as a Cathode for Sodium-Ion BatteriesOriginal File Name Searched:
nanomaterials-12-00984-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |