
PDF Publication Title:
Text from PDF Page: 009
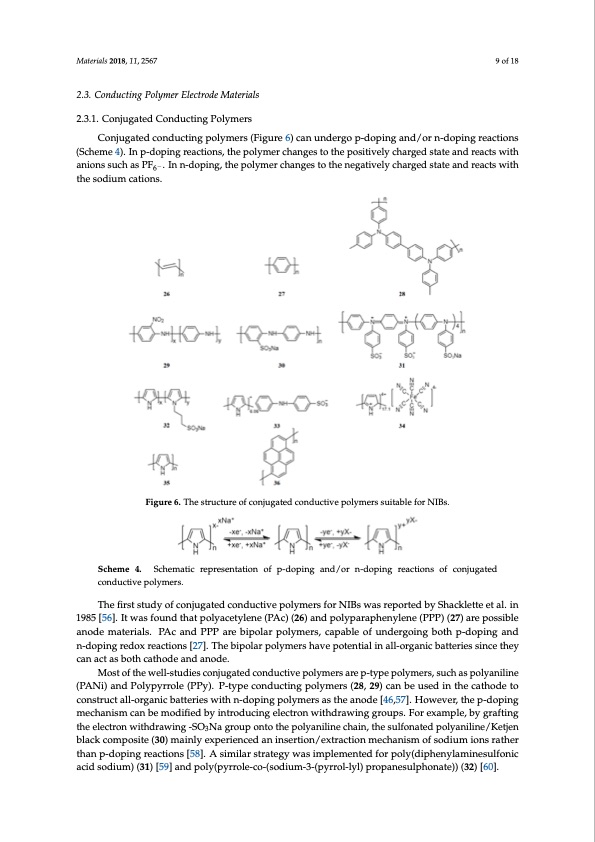
Materials 2018, 11, 2567 9 of 18 2.3. Conducting Polymer Electrode Materials 2.3.1. Conjugated Conducting Polymers Conjugated conducting polymers (Figure 6) can undergo p-doping and/or n-doping reactions (Scheme 4). In p-doping reactions, the polymer changes to the positively charged state and reacts with anions such as PF6− . In n-doping, the polymer changes to the negatively charged state and reacts with the sodium cations. Materials 2018, 11, x FOR PEER REVIEW 10 of 20 Materials 2018, 11, x FOR PEER REVIEW 9 of 20 2.3. Conducting Polymer Electrode Materials 2.3.1. Conjugated Conducting Polymers Conjugated conducting polymers (Figure 6) can undergo p‐doping and/or n‐doping reactions (Scheme 4). In p‐doping reactions, the polymer changes to the positively charged state and reacts with anions such as PF6‐. In n‐doping, the polymer changes to the negatively charged state and reacts with The advantage of conducting polymers over other polymers is their high electric conductivity comparable to semiconductors or even metals [61]. However, their capacity is low and limited by the degree of doping. Incorporation of a high‐capacity redox‐active unit can be used to overcome this drawback, as extensively studied by Yang’s group [62–64]. By grafting o‐nitroaniline groups onto polyaniline chains, polymer (29) achieved a high reversible capacity of 180 mAhꞏg−1 and retained at 173 SchSecmheeme44..SchSecmheatmicarteipcrersenptraetisoennotfapti‐odnopionfgapn-d/orpni‐ndgopainngdr/eaocrtions-doofpcoinjgugarteadcctoionndusctoivfepcolnyjmuegras.ted mAhꞏg−1 at the 50th cycle [62]. Similarly, by doping with diphenylamine sulfonate anions (33) [63] and the sodium cations. conductive polymers. Figure6Fi.gTuhree6s.TtrhuecstururcetuorfecoofcnojnujuggaattedconndducuticvteivpeolpymoleyrsmsueirtsabsluefiotarbNlIeBsf.orNIBs. ferrocyanides (34) [64], the anion‐doped polypyrroles demonstrated increased capacities due to the The first study of conjugated conductive polymers for NIBs was reported by Shacklette et al. in contribution from the doped redox‐active units. Thus, to develop high‐performance conjugated 1985 [56]. It was found that polyacetylene (PAc) (26) and polyparaphenylene (PPP) (27) are possible The first study of conjugated conductive polymers for NIBs was reported by Shacklette et al. in conductive polymer electrode materials, it is feasible and promising to focus on the incorporation of anode materials. PAc and PPP are bipolar polymers, capable of undergoing both p‐doping and n‐ 1985 [56].otIhtewr raedsofxo‐uacntidvetuhnaitspoontloyathceeptyolyemner(cPhAainc.) (26) and polyparaphenylene (PPP) (27) are possible doping redox reactions [27]. The bipolar polymers have potential in all‐organic batteries since they can It has been reported that the particle size, morphology, crystalline structure of conjugated anode materials. PAc and PPP are bipolar polymers, capable of undergoing both p-doping and act as both cathode and anode. conductive polymers may affect their electrochemical performance. Wang’s group [65] found that n-doping redox reactions [27]. The bipolar polymers have potential in all-organic batteries since they Most of the well‐studies conjugated conductive polymers are p‐type polymers, such as polyaniline submicron polypyrrole (35) with fluffy structure and chain‐like morphology showed a higher capacity canact(PaAsNboi)thancdatPholydpeyarrnodlea(nPPoyd)e..P‐typeconductin−1gpolymers(28,29)canbeusedinthecathodeto (183mAhꞏg−o1)thanbulkpolypyrrole(34.8mAhꞏg ).Theexcellentelectrochemicalperformanceisdue Mcoosntosottrfhutechtueanlwilq‐uoeerlgl-sastnrtuicdtbuiareetst, ecwroiheniscjuhwgintahctrenda‐sdceodopntihndeguecpletoiclvtyremicpaelorscloyanmstatechtresbaeantrwoeedpen-[tt4yh6pe,5ep7op].loyHplyoymwrroelvreespr,,asrtuhticechlepsa‐dasnopdpoinlyganiline mepcrhoamnoistmedctahne bpenmetordatiifoiendobf ythienetrleocdtruocliynteg ienlteoctrhoenmwaittehridarl.aIwn iandgdgitrioun,phso. lFlowr enxanmopspleh,ebreysg[r6a6f]taingd the (PANi) and Polypyrrole (PPy). P-type conducting polymers (28, 29) can be used in the cathode to elemcterosonpworiothudsrnaawnionsghe‐SeOts3N[67a]gorof uppoloynptyorrtohlepwoelyreanpilrienpeacrehdainth,rtohuegshultfhoenautsedopfosloyfatntielimnpe/lKatetsj.enThbelack construct all-organic batteries with n-doping polymers as the anode [46,57]. However, the p-doping unique morphology enabled stable cycling performance and superior rate capability of the polypyrrole composite (30) mainly experienced an insertion/extraction mechanism of sodium ions rather than p‐ mechanism can be modified by introducing electron withdrawing groups. For example, by grafting electrodes. Han et al. [68] reported crystalline and amorphous oligopyrenes (36) as high‐voltage doping reactions [58]. A similar strategy was implemented for poly(diphenylaminesulfonic acid theelectrcoanthwodiethsdforraswodiniugm-‐SioOnbNatategrireos.uTpheoncrtyostahlleinpeolyigaonpiylrienneecwhiathina,tlahyeerseudlfsotrnuactuerdep(Foiglyuareni7lai)ne/Ketjen sodium) (31) [59] and poly3(pyrrole‐co‐(sodium‐3‐(pyrrol‐lyl) propanesulphonate)) (32) [60]. exhibited sloping charge and discharge curves with a discharge capacity of 42.5 mAhꞏg−1 and an black composite (30) mainly experienced an insertion/extraction mechanism of sodium ions rather than p-doping reactions [58]. A similar strategy was implemented for poly(diphenylaminesulfonic acid sodium) (31) [59] and poly(pyrrole-co-(sodium-3-(pyrrol-lyl) propanesulphonate)) (32) [60].PDF Image | Polymer Electrode Materials for Sodium-ion Batteries
PDF Search Title:
Polymer Electrode Materials for Sodium-ion BatteriesOriginal File Name Searched:
materials-11-02567.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |