
PDF Publication Title:
Text from PDF Page: 010
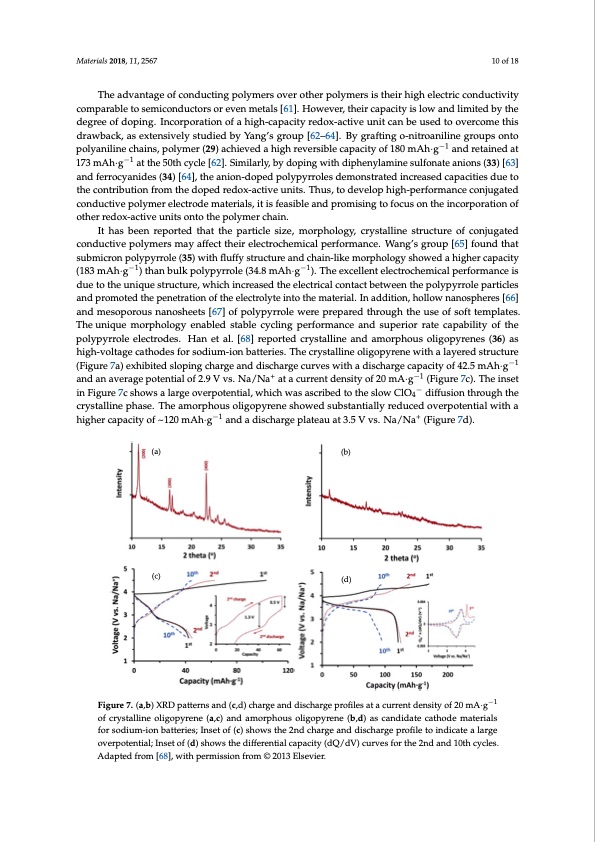
Materials 2018, 11, 2567 10 of 18 The advantage of conducting polymers over other polymers is their high electric conductivity comparable to semiconductors or even metals [61]. However, their capacity is low and limited by the degree of doping. Incorporation of a high-capacity redox-active unit can be used to overcome this drawback, as extensively studied by Yang’s group [62–64]. By grafting o-nitroaniline groups onto polyaniline chains, polymer (29) achieved a high reversible capacity of 180 mAh·g−1 and retained at 173 mAh·g−1 at the 50th cycle [62]. Similarly, by doping with diphenylamine sulfonate anions (33) [63] and ferrocyanides (34) [64], the anion-doped polypyrroles demonstrated increased capacities due to the contribution from the doped redox-active units. Thus, to develop high-performance conjugated conductive polymer electrode materials, it is feasible and promising to focus on the incorporation of other redox-active units onto the polymer chain. It has been reported that the particle size, morphology, crystalline structure of conjugated conductive polymers may affect their electrochemical performance. Wang’s group [65] found that submicron polypyrrole (35) with fluffy structure and chain-like morphology showed a higher capacity (183 mAh·g−1) than bulk polypyrrole (34.8 mAh·g−1). The excellent electrochemical performance is due to the unique structure, which increased the electrical contact between the polypyrrole particles and promoted the penetration of the electrolyte into the material. In addition, hollow nanospheres [66] and mesoporous nanosheets [67] of polypyrrole were prepared through the use of soft templates. The unique morphology enabled stable cycling performance and superior rate capability of the polypyrrole electrodes. Han et al. [68] reported crystalline and amorphous oligopyrenes (36) as high-voltage cathodes for sodium-ion batteries. The crystalline oligopyrene with a layered structure (Figure 7a) exhibited sloping charge and discharge curves with a discharge capacity of 42.5 mAh·g−1 Materials 2018, 11, x FOR PEER REVIEW 11 of 20 and an average potential of 2.9 V vs. Na/Na+ at a current density of 20 mA·g−1 (Figure 7c). The inset average potential of 2.9 V vs. Na/Na+ at a current density of 20 mAꞏg−1 (Figure 7c)−. The inset in Figure in Figure 7c shows a large overpotential, which was ascribed to the slow ClO4 diffusion through the 7c shows a large overpotential, which was ascribed to the slow ClO4− diffusion through the crystalline crystalline phase. The amorphous oligopyrene showed substantially reduced overpotential with a phase. The amorphous oligopyrene showed substantially reduced overpotential with a higher capacity higher capacity of ~120 mAh·g−1 and a discharge plateau at 3.5 V vs. Na/Na+ (Figure 7d). of ~120 mAh g−1 and a discharge plateau at 3.5 V vs. Na/Na+ (Figure 7d). (a) (b) (c) (d) Figure7.(a,b)XRDpatternsand(c,d)chargeanddischargeprofilesatacurrentdensityof20−m1A·g−1 Figure 7. (a,b) XRD patterns and (c,d) charge and discharge profiles at a current density of 20 mAꞏg of of crystalline oligopyrene (a,c) and amorphous oligopyrene (b,d) as candidate cathode materials crystalline oligopyrene (a,c) and amorphous oligopyrene (b,d) as candidate cathode materials for for sodiuiumm‐i-oinonbabtatettreiersi;esIn; sIentseotf o(fc)(cs)hsohwoswthseth2end2ncdhacrhgaerganedanddiscdhiasrcghearpgreofpilreotfioleintdoicinatdeicaatlaeragelarge oveorvpeortpeontteinatli;aIln; Isnesteotfo(fd()d)shshoowwssththeediifferentiallcapaaccitiyty(d(dQQ/d/Vd)Vcu)rcvuersvfeosr ftohre t2hned2and a1n0tdh1c0ytchlecs.ycles. Adapted from [68], with permission from © 2013 Elsevier. Adapted from [68], with permission from © 2013 Elsevier. 2.3.2. Non‐Conjugated Conductive Radical Polymers The most widely‐explored organic radical polymers for batteries are nitroxide radical polymers (Figure 8). The nitroxide radical polymers can be not only reversibly n‐doped to aminoxy anions in cathodic reactions at relatively high voltage but also p‐doped to oxoammonium cations in anodic reactions at relative low voltage (Scheme 5).PDF Image | Polymer Electrode Materials for Sodium-ion Batteries
PDF Search Title:
Polymer Electrode Materials for Sodium-ion BatteriesOriginal File Name Searched:
materials-11-02567.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |