
PDF Publication Title:
Text from PDF Page: 016
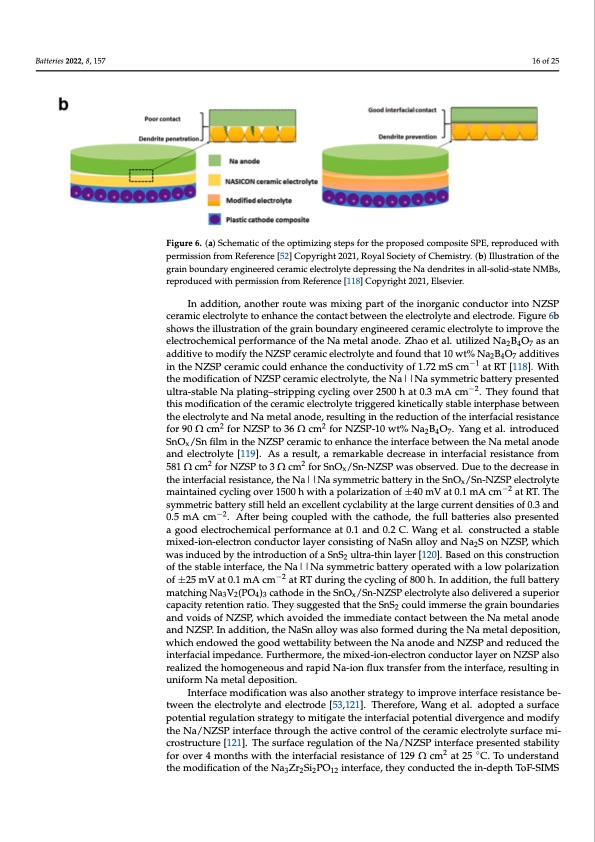
Batteries 2022, 8, 157 16 of 25 FigFuirgeur6e. 6(a.)(aS)cShcehmemataitcicooffttheoptimiziingssteteppssfofrotrhtehperoppropseodsecdomcpoomsipteosSiPteE,SrPeEpr,ordeupcreoduwcitehd with permission from Reference [52] Copyright 2021, Royal Society of Chemistry. (b) Illustration of the permission from Reference [52] Copyright 2021, Royal Society of Chemistry. (b) Illustration of the grain boundary engineered ceramic electrolyte depressing the Na dendrites in all-solid-state NMBs, grain boundary engineered ceramic electrolyte depressing the Na dendrites in all-solid-state NMBs, reproduced with permission from Reference [118] Copyright 2021, Elsevier. reproduced with permission from Reference [118] Copyright 2021, Elsevier. In addition, another route was mixing part of the inorganic conductor into NZSP In addition, another route was mixing part of the inorganic conductor into NZSP ceramic electrolyte to enhance the contact between the electrolyte and electrode. Figure 6b ceramic electrolyte to enhance the contact between the electrolyte and electrode. Figure shows the illustration of the grain boundary engineered ceramic electrolyte to improve the 6b shows the illustration of the grain boundary engineered ceramic electrolyte to improve electrochemical performance of the Na metal anode. Zhao et al. utilized Na2B4O7 as an theaedldeicttivroe ctohemoidciafyl ptheerfNoZrmSPacnecreamoficthelecNtraolmytetanldafnooudned. tZhhata1o0ewt ta%l. NutailBizeOd Nadad2Biti4vOe7s as an 247 in the NZSP ceramic could enhance the conductivity of 1.72 mS cm−1 at RT [118]. With additive to modify the NZSP ceramic electrolyte and found that 10 wt% Na2B4O7 additives the modification of NZSP ceramic electrolyte, the Na||Na symmetric−1battery presented in the NZSP ceramic could enhance the conductivity of 1.72 mS cm at RT [118]. With the ultra-stable Na plating–stripping cycling over 2500 h at 0.3 mA cm−2. They found that modification of NZSP ceramic electrolyte, the Na||Na symmetric battery presented ultra- this modification of the ceramic electrolyte triggered kinetically stable interphase between stable Na plating–stripping cycling over 2500 h at 0.3 mA cm−2. They found that this mod- the electrolyte and Na metal anode, resulting in the reduction of the interfacial resistance ification of the2ceramic electrolyte tr2iggered kinetically stable interphase between the elec- for 90 Ω cm for NZSP to 36 Ω cm for NZSP-10 wt% Na2B4O7. Yang et al. introduced trolyte and Na metal anode, resulting in the reduction of the interfacial resistance for 90 SnOx/Sn film in the NZSP ceramic to enhance the interface between the Na metal anode Ω cm2 for NZSP to 36 Ω cm2 for NZSP-10 wt% Na2B4O7. Yang et al. introduced SnOx/Sn and electrolyte [119]. As a result, a remarkable decrease in interfacial resistance from 22 film58i1nΩthcemNfZoSrPNcZeSrPamtoi3cΩtocemnhfaonrcSenOthe/Sint-eNrZfaScPewbeastwobeseenrvtehde.NDauemtoetahleadneocrdeeasaenidn elec- x the interfacial resistance, the Na||Na symmetric battery in the SnO /Sn-NZSP electrolyte 2 trolyte [119]. As a result, a remarkable decrease in interfacial rexsistance from 581 Ω cm maintained cycling2 over 1500 h with a polarization of ±40 mV at 0.1 mA cm−2 at RT. The for NZSP to 3 Ω cm for SnOx/Sn-NZSP was observed. Due to the decrease in the interfa- symmetric battery still held an excellent cyclability at the large current densities of 0.3 and cial resistance, the Na||Na symmetric battery in the SnOx/Sn-NZSP electrolyte main- 0.5 mA cm−2. After being coupled with the cathode, the full batteries also presented tained cycling over 1500 h with a polarization of ±40 mV at 0.1 mA cm−2 at RT. The sym- a good electrochemical performance at 0.1 and 0.2 C. Wang et al. constructed a stable metric battery still held an excellent cyclability at the large current densities of 0.3 and 0.5 mixed-ion-electron conductor layer consisting of NaSn alloy and Na2S on NZSP, which mA cm–2. After being coupled with the cathode, the full batteries also presented a good was induced by the introduction of a SnS2 ultra-thin layer [120]. Based on this construction electrochemical performance at 0.1 and 0.2 C. Wang et al. constructed a stable mixed-ion- of the stable interface, the Na||Na symmetric battery operated with a low polarization −2 eleoctfr±on25cmonVdautc0t.o1rmlaAyecrmconastisRtTindguorifnNgathSencaylclloinygaonfd8N00ah2S. IonnadNdZitSioPn,,wtheicfuhllwbasttienryduced matching Na V (PO ) cathode in the SnO /Sn-NZSP electrolyte also delivered a superior by the introduc3tio2 n of4 a3 SnS2 ultra-thin layxer [120]. Based on this construction of the stable capacityretentionratio.TheysuggestedthattheSnS couldimmersethegrainboundaries 2 interface, the Na||Na symmetric battery operated with a low polarization of ±25 mV at and voids of NZSP, which avoided the immediate contact between the Na metal anode 0.1 mA cm−2 at RT during the cycling of 800 h. In addition, the full battery matching and NZSP. In addition, the NaSn alloy was also formed during the Na metal deposition, Na3V2(PO4)3 cathode in the SnOx/Sn-NZSP electrolyte also delivered a superior capacity which endowed the good wettability between the Na anode and NZSP and reduced the retention ratio. They suggested that the SnS2 could immerse the grain boundaries and interfacial impedance. Furthermore, the mixed-ion-electron conductor layer on NZSP also realized the homogeneous and rapid Na-ion flux transfer from the interface, resulting in uniform Na metal deposition. Interface modification was also another strategy to improve interface resistance be- tween the electrolyte and electrode [53,121]. Therefore, Wang et al. adopted a surface potential regulation strategy to mitigate the interfacial potential divergence and modify the Na/NZSP interface through the active control of the ceramic electrolyte surface mi- crostructure [121]. The surface regulation of the Na/NZSP interface presented stability for over 4 months with the interfacial resistance of 129 Ω cm2 at 25 ◦C. To understand the modification of the Na3Zr2Si2PO12 interface, they conducted the in-depth ToF-SIMSPDF Image | Recent Development for Sodium Metal Batteries
PDF Search Title:
Recent Development for Sodium Metal BatteriesOriginal File Name Searched:
batteries-08-00157-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |