
PDF Publication Title:
Text from PDF Page: 017
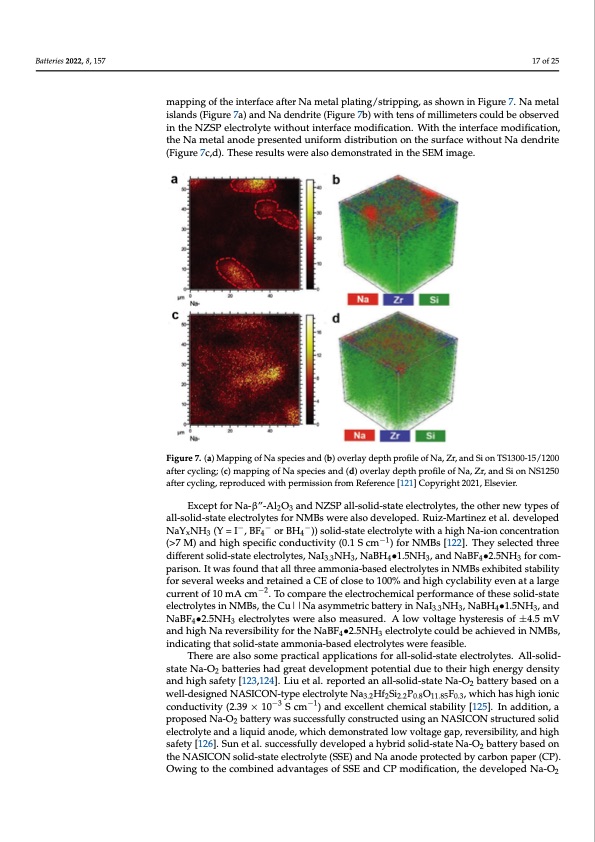
Batteries 2022, 8, 157 tween the electrolyte and electrode [53,121]. Therefore, Wang et al. adopted a surface po tential regulation strategy to mitigate the interfacial potential divergence and modify th Na/NZSP interface through the active control of the ceramic electrolyte surface micro structure [121]. The surface regulation of the Na/ NZSP interface presented stability fo 2 17 of 25 over 4 months with the interfacial resistance of 129 Ω cm at 25 °C. To understand th modification of the Na3Zr2Si2PO12 interface, they conducted the in-depth ToF-SIMS map ping of the interface after Na metal plating/stripping, as shown in Figure 7. Na metal is mapping of the interface after Na metal plating/stripping, as shown in Figure 7. Na metal lands (Figure 7a) and Na dendrite (Figure 7b) with tens of millimeters could be observe islands (Figure 7a) and Na dendrite (Figure 7b) with tens of millimeters could be observed in the NZSP electrolyte without interface modification. With the interface modification in the NZSP electrolyte without interface modification. With the interface modification, the Na metal anode presented uniform distribution on the surface without Na dendrit the Na metal anode presented uniform distribution on the surface without Na dendrite (Figure 7c,d). These results were also demonstrated in the SEM image. (Figure 7c,d). These results were also demonstrated in the SEM image. Figure 7. (a) Mapping of Na species and (b) overlay depth profile of Na, Zr, and Si on TS1300-15/1200 Figure 7. (a) Mapping of Na species and (b) overlay depth profile of Na, Zr, and Si on TS1300 after cycling; (c) mapping of Na species and (d) overlay depth profile of Na, Zr, and Si on NS1250 15/1200 after cycling; (c) mapping of Na species and (d) overlay depth profile of Na, Zr, and Si o after cycling, reproduced with permission from Reference [121] Copyright 2021, Elsevier. NS1250 after cycling, reproduced with permission from Reference [121] Copyright 2021, Elsevier. Except for Na-β”-Al2O3 and NZSP all-solid-state electrolytes, the other new types of Except for Na-β”-Al2O3 and NZSP all-solid-state electrolytes, the other new types o all-solid-state electrolytes for NMBs were also developed. Ruiz-Martinez et al. developed −−− aNlal-YsoNliHd-s(tYat=e eI le, cBtFrolyoter sBHfor N))MsoBlisd-wstearte eallescotrodleyvtelwoiptheda.hRiguhizN-Ma-iaornticnoenzcenttaral.tidoenvelope x344 −1 N(>a7YMxN)aHn3d(Yhig=hIs,pBeFc4ifiocrcBonHd4u)c)tisvoiltiyd(-0s.t1atSecemlect)rofolyrtNeMwiBtsh[a12h2i]g.hTNheay-isoenlectoendctehnrtereation(> −−− differentsolid-stateelectrolytes,NaINH,Na−B1H•1.5NH,andNaBF•2.5NHforcom- M)andhighspecificconductivity3.(30.1S3cm )fo4rNMB3s[122].The4yselecte3dthreedifferen parison. It was found that all three ammonia-based electrolytes in NMBs exhibited stability solid-state electrolytes, NaI3.3NH3, NaBH4•1.5NH3, and NaBF4•2.5NH3 for comparison. I for several weeks and retained a CE of close to 100% and high cyclability even at a large was found that all three ammonia-based electrolytes in NMBs exhibited stability for sev current of 10 mA cm−2. To compare the electrochemical performance of these solid-state eral weeks and retained a CE of close to 100% and high cyclability even at a large curren electrolytes in NMBs, the Cu||Na asymmetric battery in NaI3.3NH3, NaBH4•1.5NH3, and of 10 mA cm−2. To compare the electrochemical performance of these solid-state electro NaBF4•2.5NH3 electrolytes were also measured. A low voltage hysteresis of ±4.5 mV lytes in NMBs, the Cu||Na asymmetric battery in NaI3.3NH3, NaBH4•1.5NH3, an and high Na reversibility for the NaBF4•2.5NH3 electrolyte could be achieved in NMBs, NaBF4•2.5NH3 electrolytes were also measured. A low voltage hysteresis of ±4.5 mV an indicating that solid-state ammonia-based electrolytes were feasible. There are also some practical applications for all-solid-state electrolytes. All-solid- state Na-O2 batteries had great development potential due to their high energy density and high safety [123,124]. Liu et al. reported an all-solid-state Na-O2 battery based on a well-designed NASICON-type electrolyte Na3.2Hf2Si2.2P0.8O11.85F0.3, which has high ionic conductivity (2.39 × 10−3 S cm−1) and excellent chemical stability [125]. In addition, a proposed Na-O2 battery was successfully constructed using an NASICON structured solid electrolyte and a liquid anode, which demonstrated low voltage gap, reversibility, and high safety [126]. Sun et al. successfully developed a hybrid solid-state Na-O2 battery based on the NASICON solid-state electrolyte (SSE) and Na anode protected by carbon paper (CP). Owing to the combined advantages of SSE and CP modification, the developed Na-O2 - e - r e - - d e - n f d 7 t t - t - d dPDF Image | Recent Development for Sodium Metal Batteries
PDF Search Title:
Recent Development for Sodium Metal BatteriesOriginal File Name Searched:
batteries-08-00157-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |