
PDF Publication Title:
Text from PDF Page: 005
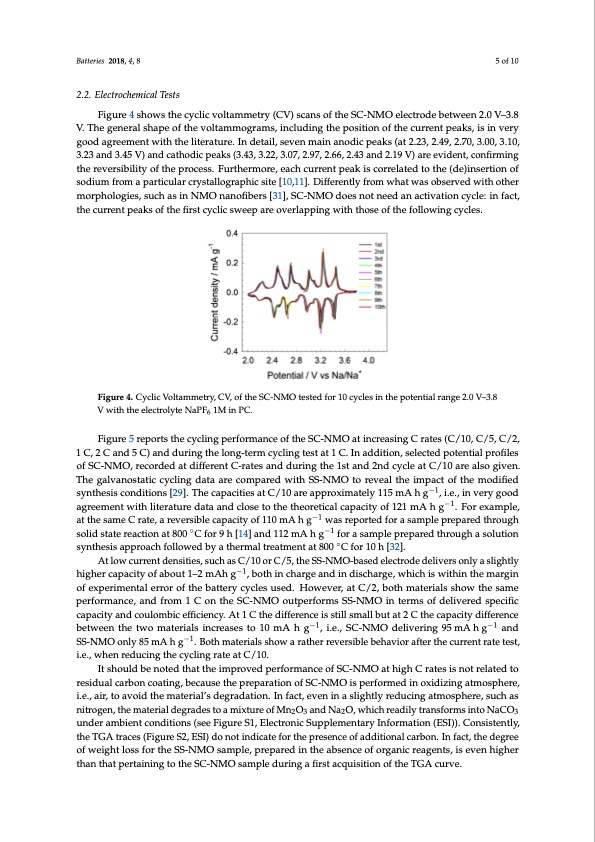
Batteries 2018, 4, 8 5 of 10 2.2. Electrochemical Tests Figure 4 shows the cyclic voltammetry (CV) scans of the SC-NMO electrode between 2.0 V–3.8 V. The general shape of the voltammograms, including the position of the current peaks, is in very good agreement with the literature. In detail, seven main anodic peaks (at 2.23, 2.49, 2.70, 3.00, 3.10, 3.23 and 3.45 V) and cathodic peaks (3.43, 3.22, 3.07, 2.97, 2.66, 2.43 and 2.19 V) are evident, confirming the reversibility of the process. Furthermore, each current peak is correlated to the (de)insertion of sodium from a particular crystallographic site [10,11]. Differently from what was observed with other morphologies, such as in NMO nanofibers [31], SC-NMO does not need an activation cycle: in fact, the current peaks of the first cyclic sweep are overlapping with those of the following cycles. Figure 4. Cyclic Voltammetry, CV, of the SC-NMO tested for 10 cycles in the potential range 2.0 V–3.8 V with the electrolyte NaPF6 1M in PC. Figure 5 reports the cycling performance of the SC-NMO at increasing C rates (C/10, C/5, C/2, 1 C, 2 C and 5 C) and during the long-term cycling test at 1 C. In addition, selected potential profiles of SC-NMO, recorded at different C-rates and during the 1st and 2nd cycle at C/10 are also given. The galvanostatic cycling data are compared with SS-NMO to reveal the impact of the modified synthesis conditions [29]. The capacities at C/10 are approximately 115 mA h g−1, i.e., in very good agreement with literature data and close to the theoretical capacity of 121 mA h g−1. For example, at the same C rate, a reversible capacity of 110 mA h g−1 was reported for a sample prepared through solid state reaction at 800 ◦C for 9 h [14] and 112 mA h g−1 for a sample prepared through a solution synthesis approach followed by a thermal treatment at 800 ◦C for 10 h [32]. At low current densities, such as C/10 or C/5, the SS-NMO-based electrode delivers only a slightly higher capacity of about 1–2 mAh g−1, both in charge and in discharge, which is within the margin of experimental error of the battery cycles used. However, at C/2, both materials show the same performance, and from 1 C on the SC-NMO outperforms SS-NMO in terms of delivered specific capacity and coulombic efficiency. At 1 C the difference is still small but at 2 C the capacity difference between the two materials increases to 10 mA h g−1, i.e., SC-NMO delivering 95 mA h g−1 and SS-NMO only 85 mA h g−1. Both materials show a rather reversible behavior after the current rate test, i.e., when reducing the cycling rate at C/10. It should be noted that the improved performance of SC-NMO at high C rates is not related to residual carbon coating, because the preparation of SC-NMO is performed in oxidizing atmosphere, i.e., air, to avoid the material’s degradation. In fact, even in a slightly reducing atmosphere, such as nitrogen, the material degrades to a mixture of Mn2O3 and Na2O, which readily transforms into NaCO3 under ambient conditions (see Figure S1, Electronic Supplementary Information (ESI)). Consistently, the TGA traces (Figure S2, ESI) do not indicate for the presence of additional carbon. In fact, the degree of weight loss for the SS-NMO sample, prepared in the absence of organic reagents, is even higher than that pertaining to the SC-NMO sample during a first acquisition of the TGA curve.PDF Image | Sodium-Ion Batteries Obtained through Urea Based
PDF Search Title:
Sodium-Ion Batteries Obtained through Urea BasedOriginal File Name Searched:
10281-250332.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |