
PDF Publication Title:
Text from PDF Page: 056
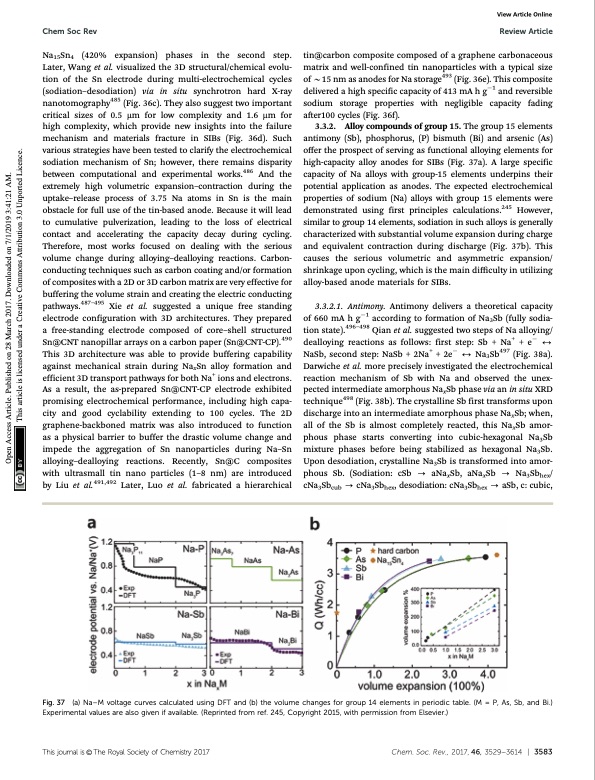
Chem Soc Rev Review Article tin@carbon composite composed of a graphene carbonaceous matrix and well-confined tin nanoparticles with a typical size of B15 nm as anodes for Na storage493 (Fig. 36e). This composite delivered a high specific capacity of 413 mA h g1 and reversible sodium storage properties with negligible capacity fading after100 cycles (Fig. 36f). 3.3.2. Alloy compounds of group 15. The group 15 elements antimony (Sb), phosphorus, (P) bismuth (Bi) and arsenic (As) offer the prospect of serving as functional alloying elements for high-capacity alloy anodes for SIBs (Fig. 37a). A large specific capacity of Na alloys with group-15 elements underpins their potential application as anodes. The expected electrochemical properties of sodium (Na) alloys with group 15 elements were demonstrated using first principles calculations.245 However, similar to group 14 elements, sodiation in such alloys is generally characterized with substantial volume expansion during charge and equivalent contraction during discharge (Fig. 37b). This causes the serious volumetric and asymmetric expansion/ shrinkage upon cycling, which is the main difficulty in utilizing alloy-based anode materials for SIBs. 3.3.2.1. Antimony. Antimony delivers a theoretical capacity of 660 mA h g1 according to formation of Na3Sb (fully sodia- tion state).496–498 Qian et al. suggested two steps of Na alloying/ dealloying reactions as follows: first step: Sb + Na+ + e 2 NaSb, second step: NaSb + 2Na+ + 2e 2 Na3Sb497 (Fig. 38a). Darwiche et al. more precisely investigated the electrochemical reaction mechanism of Sb with Na and observed the unex- pected intermediate amorphous NaxSb phase via an in situ XRD technique498 (Fig. 38b). The crystalline Sb first transforms upon discharge into an intermediate amorphous phase NaxSb; when, all of the Sb is almost completely reacted, this NaxSb amor- phous phase starts converting into cubic-hexagonal Na3Sb mixture phases before being stabilized as hexagonal Na3Sb. Upon desodiation, crystalline Na3Sb is transformed into amor- phous Sb. (Sodiation: cSb - aNaxSb, aNaxSb - Na3Sbhex/ cNa3Sbcub - cNa3Sbhex, desodiation: cNa3Sbhex - aSb, c: cubic, Na15Sn4 (420% expansion) phases in the second step. Later, Wang et al. visualized the 3D structural/chemical evolu- tion of the Sn electrode during multi-electrochemical cycles (sodiation–desodiation) via in situ synchrotron hard X-ray nanotomography485 (Fig. 36c). They also suggest two important critical sizes of 0.5 mm for low complexity and 1.6 mm for high complexity, which provide new insights into the failure mechanism and materials fracture in SIBs (Fig. 36d). Such various strategies have been tested to clarify the electrochemical sodiation mechanism of Sn; however, there remains disparity between computational and experimental works.486 And the extremely high volumetric expansion–contraction during the uptake–release process of 3.75 Na atoms in Sn is the main obstacle for full use of the tin-based anode. Because it will lead to cumulative pulverization, leading to the loss of electrical contact and accelerating the capacity decay during cycling. Therefore, most works focused on dealing with the serious volume change during alloying–dealloying reactions. Carbon- conducting techniques such as carbon coating and/or formation of composites with a 2D or 3D carbon matrix are very effective for buffering the volume strain and creating the electric conducting pathways.487–495 Xie et al. suggested a unique free standing electrode configuration with 3D architectures. They prepared a free-standing electrode composed of core–shell structured Sn@CNT nanopillar arrays on a carbon paper (Sn@CNT-CP).490 This 3D architecture was able to provide buffering capability against mechanical strain during NaxSn alloy formation and efficient 3D transport pathways for both Na+ ions and electrons. As a result, the as-prepared Sn@CNT-CP electrode exhibited promising electrochemical performance, including high capa- city and good cyclability extending to 100 cycles. The 2D graphene-backboned matrix was also introduced to function as a physical barrier to buffer the drastic volume change and impede the aggregation of Sn nanoparticles during Na–Sn alloying–dealloying reactions. Recently, Sn@C composites with ultrasmall tin nano particles (1–8 nm) are introduced by Liu et al.491,492 Later, Luo et al. fabricated a hierarchical View Article Online Fig. 37 (a) Na–M voltage curves calculated using DFT and (b) the volume changes for group 14 elements in periodic table. (M = P, As, Sb, and Bi.) Experimental values are also given if available. (Reprinted from ref. 245, Copyright 2015, with permission from Elsevier.) Thisjournalis©TheRoyalSocietyofChemistry2017 Chem.Soc.Rev.,2017,46,3529--3614 | 3583 Open Access Article. Published on 28 March 2017. Downloaded on 7/1/2019 3:41:21 AM. This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.PDF Image | Sodium-ion batteries present and future
PDF Search Title:
Sodium-ion batteries present and futureOriginal File Name Searched:
Sodium-ion batteries present and future.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |