
PDF Publication Title:
Text from PDF Page: 061
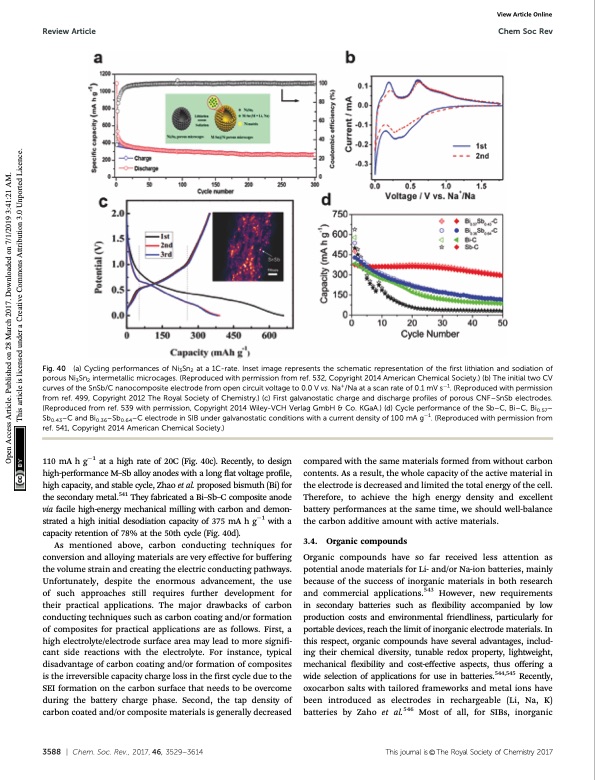
Review Article Chem Soc Rev Fig. 40 (a) Cycling performances of Ni3Sn2 at a 1C-rate. Inset image represents the schematic representation of the first lithiation and sodiation of porous Ni3Sn2 intermetallic microcages. (Reproduced with permission from ref. 532, Copyright 2014 American Chemical Society.) (b) The initial two CV curves of the SnSb/C nanocomposite electrode from open circuit voltage to 0.0 V vs. Na+/Na at a scan rate of 0.1 mV s1. (Reproduced with permission from ref. 499, Copyright 2012 The Royal Society of Chemistry.) (c) First galvanostatic charge and discharge profiles of porous CNF–SnSb electrodes. (Reproduced from ref. 539 with permission, Copyright 2014 Wiley-VCH Verlag GmbH & Co. KGaA.) (d) Cycle performance of the Sb–C, Bi–C, Bi0.57– Sb0.43–C and Bi0.36–Sb0.64–C electrode in SIB under galvanostatic conditions with a current density of 100 mA g1. (Reproduced with permission from ref. 541, Copyright 2014 American Chemical Society.) View Article Online 110 mA h g1 at a high rate of 20C (Fig. 40c). Recently, to design high-performance M–Sb alloy anodes with a long flat voltage profile, high capacity, and stable cycle, Zhao et al. proposed bismuth (Bi) for the secondary metal.541 They fabricated a Bi–Sb–C composite anode via facile high-energy mechanical milling with carbon and demon- strated a high initial desodiation capacity of 375 mA h g1 with a capacity retention of 78% at the 50th cycle (Fig. 40d). As mentioned above, carbon conducting techniques for conversion and alloying materials are very effective for buffering the volume strain and creating the electric conducting pathways. Unfortunately, despite the enormous advancement, the use of such approaches still requires further development for their practical applications. The major drawbacks of carbon conducting techniques such as carbon coating and/or formation of composites for practical applications are as follows. First, a high electrolyte/electrode surface area may lead to more signifi- cant side reactions with the electrolyte. For instance, typical disadvantage of carbon coating and/or formation of composites is the irreversible capacity charge loss in the first cycle due to the SEI formation on the carbon surface that needs to be overcome during the battery charge phase. Second, the tap density of carbon coated and/or composite materials is generally decreased compared with the same materials formed from without carbon contents. As a result, the whole capacity of the active material in the electrode is decreased and limited the total energy of the cell. Therefore, to achieve the high energy density and excellent battery performances at the same time, we should well-balance the carbon additive amount with active materials. 3.4. Organic compounds Organic compounds have so far received less attention as potential anode materials for Li- and/or Na-ion batteries, mainly because of the success of inorganic materials in both research and commercial applications.543 However, new requirements in secondary batteries such as flexibility accompanied by low production costs and environmental friendliness, particularly for portable devices, reach the limit of inorganic electrode materials. In this respect, organic compounds have several advantages, includ- ing their chemical diversity, tunable redox property, lightweight, mechanical flexibility and cost-effective aspects, thus offering a wide selection of applications for use in batteries.544,545 Recently, oxocarbon salts with tailored frameworks and metal ions have been introduced as electrodes in rechargeable (Li, Na, K) batteries by Zaho et al.546 Most of all, for SIBs, inorganic 3588 | Chem. Soc. Rev., 2017, 46, 3529--3614 This journal is © The Royal Society of Chemistry 2017 Open Access Article. Published on 28 March 2017. Downloaded on 7/1/2019 3:41:21 AM. This article is licensed under a Creative Commons Attribution 3.0 Unported Licence.PDF Image | Sodium-ion batteries present and future
PDF Search Title:
Sodium-ion batteries present and futureOriginal File Name Searched:
Sodium-ion batteries present and future.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Salt water flow battery technology with low cost and great energy density that can be used for power storage and thermal storage. Let us de-risk your production using our license. Our aqueous flow battery is less cost than Tesla Megapack and available faster. Redox flow battery. No membrane needed like with Vanadium, or Bromine. Salgenx flow battery
| CONTACT TEL: 608-238-6001 Email: greg@salgenx.com | RSS | AMP |