
PDF Publication Title:
Text from PDF Page: 009
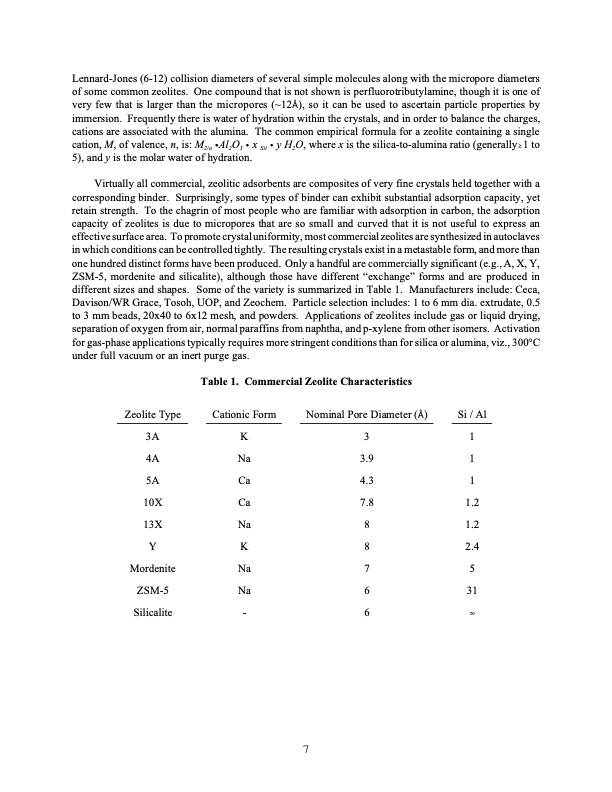
Lennard-Jones (6-12) collision diameters of several simple molecules along with the micropore diameters of some common zeolites. One compound that is not shown is perfluorotributylamine, though it is one of very few that is larger than the micropores (~12D), so it can be used to ascertain particle properties by immersion. Frequently there is water of hydration within the crystals, and in order to balance the charges, cations are associated with the alumina. The common empirical formula for a zeolite containing a single cation, M, of valence, n, is: M2/n gAl2O3 g x Sit g y H2O, where x is the silica-to-alumina ratio (generally1 to 5), and y is the molar water of hydration. Virtually all commercial, zeolitic adsorbents are composites of very fine crystals held together with a corresponding binder. Surprisingly, some types of binder can exhibit substantial adsorption capacity, yet retain strength. To the chagrin of most people who are familiar with adsorption in carbon, the adsorption capacity of zeolites is due to micropores that are so small and curved that it is not useful to express an effective surface area. To promote crystal uniformity, most commercial zeolites are synthesized in autoclaves in which conditions can be controlled tightly. The resulting crystals exist in a metastable form, and more than one hundred distinct forms have been produced. Only a handful are commercially significant (e.g., A, X, Y, ZSM-5, mordenite and silicalite), although those have different “exchange” forms and are produced in different sizes and shapes. Some of the variety is summarized in Table 1. Manufacturers include: Ceca, Davison/WR Grace, Tosoh, UOP, and Zeochem. Particle selection includes: 1 to 6 mm dia. extrudate, 0.5 to 3 mm beads, 20x40 to 6x12 mesh, and powders. Applications of zeolites include gas or liquid drying, separation of oxygen from air, normal paraffins from naphtha, and p-xylene from other isomers. Activation for gas-phase applications typically requires more stringent conditions than for silica or alumina, viz., 300°C under full vacuum or an inert purge gas. Table 1. Commercial Zeolite Characteristics Zeolite Type Cationic Form Nominal Pore Diameter (D) Si / Al 3A K 3 1 4A Na 5A Ca 10X Ca 13X Na Y K 3.9 1 4.3 1 7.8 1.2 8 1.2 8 2.4 7 5 6 31 6 4 Mordenite ZSM-5 Silicalite Na Na - 7PDF Image | ADSORBENT SELECTION
PDF Search Title:
ADSORBENT SELECTIONOriginal File Name Searched:
AdsorbentSel1B.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |