
PDF Publication Title:
Text from PDF Page: 022
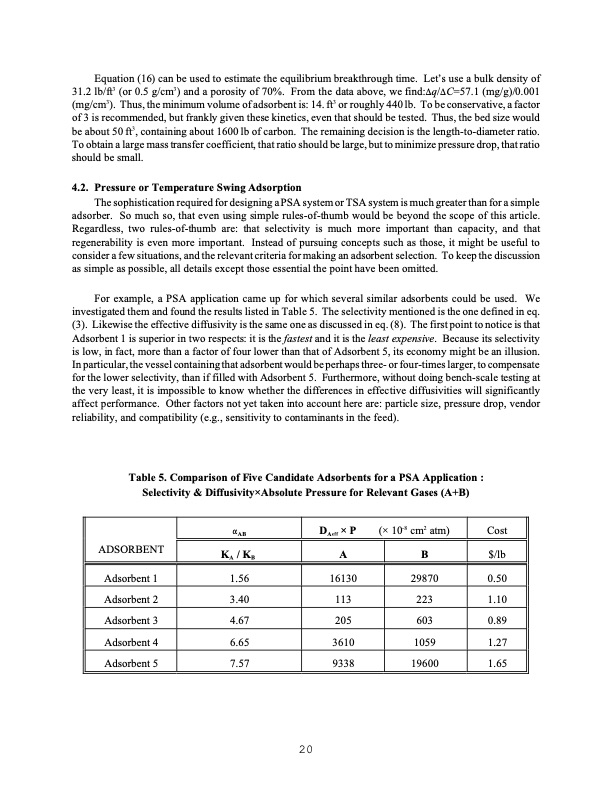
Equation (16) can be used to estimate the equilibrium breakthrough time. Let’s use a bulk density of 31.2 lb/ft3 (or 0.5 g/cm3) and a porosity of 70%. From the data above, we find:Aq/AC=57.1 (mg/g)/0.001 (mg/cm3). Thus, the minimum volume of adsorbent is: 14. ft3 or roughly 440 lb. To be conservative, a factor of 3 is recommended, but frankly given these kinetics, even that should be tested. Thus, the bed size would be about 50 ft3, containing about 1600 lb of carbon. The remaining decision is the length-to-diameter ratio. To obtain a large mass transfer coefficient, that ratio should be large, but to minimize pressure drop, that ratio should be small. 4.2. Pressure or Temperature Swing Adsorption The sophistication required for designing a PSA system or TSA system is much greater than for a simple adsorber. So much so, that even using simple rules-of-thumb would be beyond the scope of this article. Regardless, two rules-of-thumb are: that selectivity is much more important than capacity, and that regenerability is even more important. Instead of pursuing concepts such as those, it might be useful to consider a few situations, and the relevant criteria for making an adsorbent selection. To keep the discussion as simple as possible, all details except those essential the point have been omitted. For example, a PSA application came up for which several similar adsorbents could be used. We investigated them and found the results listed in Table 5. The selectivity mentioned is the one defined in eq. (3). Likewise the effective diffusivity is the same one as discussed in eq. (8). The first point to notice is that Adsorbent 1 is superior in two respects: it is the fastest and it is the least expensive. Because its selectivity is low, in fact, more than a factor of four lower than that of Adsorbent 5, its economy might be an illusion. In particular, the vessel containing that adsorbent would be perhaps three- or four-times larger, to compensate for the lower selectivity, than if filled with Adsorbent 5. Furthermore, without doing bench-scale testing at the very least, it is impossible to know whether the differences in effective diffusivities will significantly affect performance. Other factors not yet taken into account here are: particle size, pressure drop, vendor reliability, and compatibility (e.g., sensitivity to contaminants in the feed). Table 5. Comparison of Five Candidate Adsorbents for a PSA Application : Selectivity & Diffusivity×Absolute Pressure for Relevant Gases (A+B) ADSORBENT Adsorbent 1 Adsorbent 2 Adsorbent 3 Adsorbent 4 Adsorbent 5 4AB KA / KB 1.56 3.40 4.67 6.65 7.57 DAeff × P A 16130 113 205 3610 9338 (× 10-8 cm2 atm) Cost B $/lb 29870 0.50 223 1.10 603 0.89 1059 1.27 19600 1.65 20PDF Image | ADSORBENT SELECTION
PDF Search Title:
ADSORBENT SELECTIONOriginal File Name Searched:
AdsorbentSel1B.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |