
PDF Publication Title:
Text from PDF Page: 017
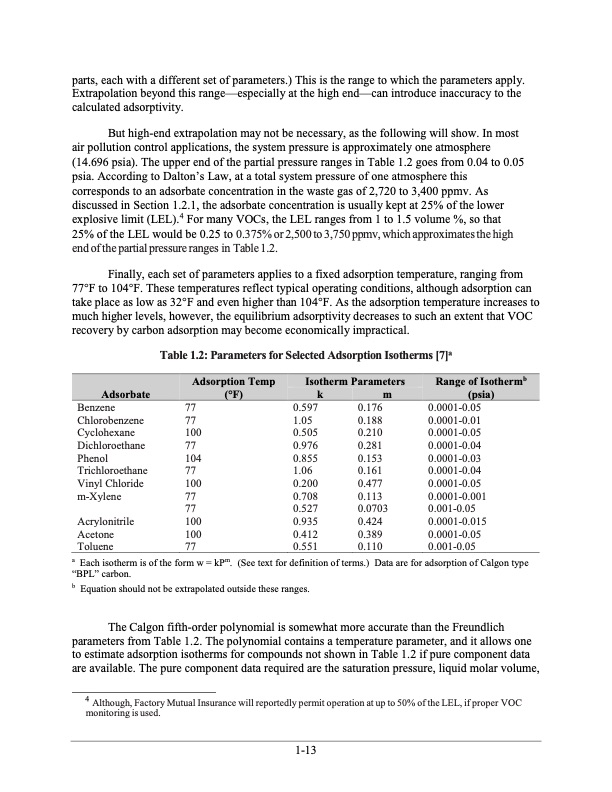
parts, each with a different set of parameters.) This is the range to which the parameters apply. Extrapolation beyond this range—especially at the high end—can introduce inaccuracy to the calculated adsorptivity. But high-end extrapolation may not be necessary, as the following will show. In most air pollution control applications, the system pressure is approximately one atmosphere (14.696 psia). The upper end of the partial pressure ranges in Table 1.2 goes from 0.04 to 0.05 psia. According to Dalton’s Law, at a total system pressure of one atmosphere this corresponds to an adsorbate concentration in the waste gas of 2,720 to 3,400 ppmv. As discussed in Section 1.2.1, the adsorbate concentration is usually kept at 25% of the lower explosive limit (LEL).4 For many VOCs, the LEL ranges from 1 to 1.5 volume %, so that 25% of the LEL would be 0.25 to 0.375% or 2,500 to 3,750 ppmv, which approximates the high end of the partial pressure ranges in Table 1.2. Finally, each set of parameters applies to a fixed adsorption temperature, ranging from 77°F to 104°F. These temperatures reflect typical operating conditions, although adsorption can take place as low as 32°F and even higher than 104°F. As the adsorption temperature increases to much higher levels, however, the equilibrium adsorptivity decreases to such an extent that VOC recovery by carbon adsorption may become economically impractical. Table 1.2: Parameters for Selected Adsorption Isotherms [7]a Adsorption Temp Adsorbate (°F) Isotherm Parameters Range of Isothermb (psia) km Benzene 77 Chlorobenzene 77 Cyclohexane 100 Dichloroethane 77 Phenol 104 Trichloroethane 77 Vinyl Chloride 100 m-Xylene 77 0.597 0.176 1.05 0.188 0.505 0.210 0.976 0.281 0.855 0.153 1.06 0.161 0.200 0.477 0.708 0.113 0.0001-0.05 0.0001-0.01 0.0001-0.05 0.0001-0.04 0.0001-0.03 0.0001-0.04 0.0001-0.05 0.0001-0.001 0.001-0.05 0.0001-0.015 0.0001-0.05 0.001-0.05 Acrylonitrile 100 Acetone 100 Toluene 77 0.935 0.424 0.412 0.389 0.551 0.110 77 0.527 0.0703 a “BPL” carbon. b Equation should not be extrapolated outside these ranges. The Calgon fifth-order polynomial is somewhat more accurate than the Freundlich parameters from Table 1.2. The polynomial contains a temperature parameter, and it allows one to estimate adsorption isotherms for compounds not shown in Table 1.2 if pure component data are available. The pure component data required are the saturation pressure, liquid molar volume, 4 Although, Factory Mutual Insurance will reportedly permit operation at up to 50% of the LEL, if proper VOC monitoring is used. Each isotherm is of the form w = kPm. (See text for definition of terms.) Data are for adsorption of Calgon type 1-13PDF Image | Carbon Adsorbers
PDF Search Title:
Carbon AdsorbersOriginal File Name Searched:
final_carbonadsorberschapter_7thedition.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |