
PDF Publication Title:
Text from PDF Page: 006
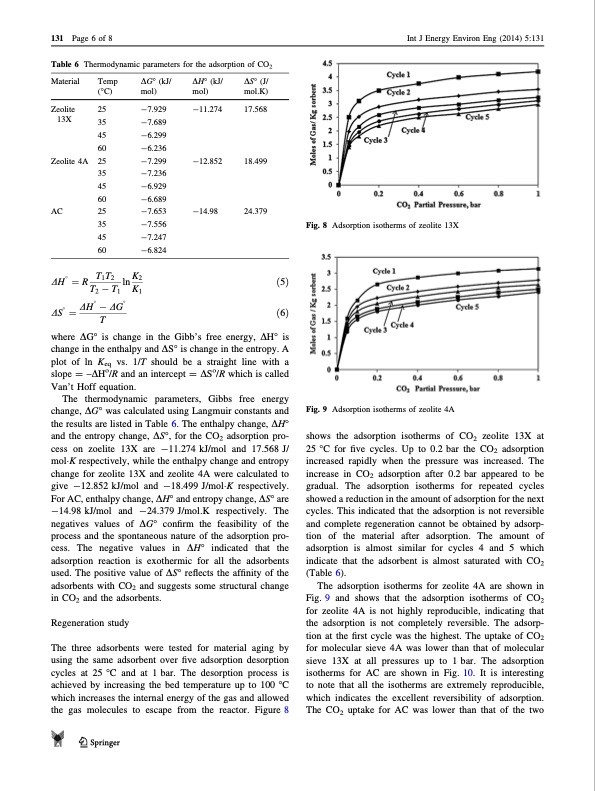
131 Page 6 of 8 Table 6 Thermodynamic parameters for the adsorption of CO2 Int J Energy Environ Eng (2014) 5:131 shows the adsorption isotherms of CO2 zeolite 13X at 25 °C for five cycles. Up to 0.2 bar the CO2 adsorption increased rapidly when the pressure was increased. The increase in CO2 adsorption after 0.2 bar appeared to be gradual. The adsorption isotherms for repeated cycles showed a reduction in the amount of adsorption for the next cycles. This indicated that the adsorption is not reversible and complete regeneration cannot be obtained by adsorp- tion of the material after adsorption. The amount of adsorption is almost similar for cycles 4 and 5 which indicate that the adsorbent is almost saturated with CO2 (Table 6). The adsorption isotherms for zeolite 4A are shown in Fig. 9 and shows that the adsorption isotherms of CO2 for zeolite 4A is not highly reproducible, indicating that the adsorption is not completely reversible. The adsorp- tion at the first cycle was the highest. The uptake of CO2 for molecular sieve 4A was lower than that of molecular sieve 13X at all pressures up to 1 bar. The adsorption isotherms for AC are shown in Fig. 10. It is interesting to note that all the isotherms are extremely reproducible, which indicates the excellent reversibility of adsorption. The CO2 uptake for AC was lower than that of the two Fig. 8 Adsorption isotherms of zeolite 13X Material Temp (°C) mol) AC 25 35 -7.556 -7.653 45 -7.247 60 -6.824 DH 1⁄4R T1T2 lnK2 ð5Þ ð6Þ DG° (kJ/ -7.929 DH° (kJ/ mol) -11.274 -12.852 -14.98 DS° (J/ mol.K) 17.568 18.499 24.379 Zeolite 25 13X Zeolite 4A 25 35 -7.236 45 -6.929 60 -6.689 35 -7.689 45 -6.299 60 -6.236 -7.299 Fig. 9 Adsorption isotherms of zeolite 4A T2 T1 K1 DH DG DS 1⁄4 T where DG° is change in the Gibb’s free energy, DH° is change in the enthalpy and DS° is change in the entropy. A plot of ln Keq vs. 1/T should be a straight line with a slope = –DHo/R and an intercept = DSo/R which is called Van’t Hoff equation. The thermodynamic parameters, Gibbs free energy change, DG° was calculated using Langmuir constants and the results are listed in Table 6. The enthalpy change, DH° and the entropy change, DS°, for the CO2 adsorption pro- cess on zoelite 13X are -11.274 kJ/mol and 17.568 J/ molK respectively, while the enthalpy change and entropy change for zeolite 13X and zeolite 4A were calculated to give -12.852 kJ/mol and -18.499 J/molK respectively. For AC, enthalpy change, DH° and entropy change, DS° are -14.98 kJ/mol and -24.379 J/mol.K respectively. The negatives values of DG° confirm the feasibility of the process and the spontaneous nature of the adsorption pro- cess. The negative values in DH° indicated that the adsorption reaction is exothermic for all the adsorbents used. The positive value of DS° reflects the affinity of the adsorbents with CO2 and suggests some structural change in CO2 and the adsorbents. Regeneration study The three adsorbents were tested for material aging by using the same adsorbent over five adsorption desorption cycles at 25 °C and at 1 bar. The desorption process is achieved by increasing the bed temperature up to 100 °C which increases the internal energy of the gas and allowed the gas molecules to escape from the reactor. Figure 8 123PDF Image | Carbon dioxide adsorption on zeolites and activated carbon PSA
PDF Search Title:
Carbon dioxide adsorption on zeolites and activated carbon PSAOriginal File Name Searched:
Carbon_dioxide_adsorption_on_zeolites_and_activate.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |