
PDF Publication Title:
Text from PDF Page: 005
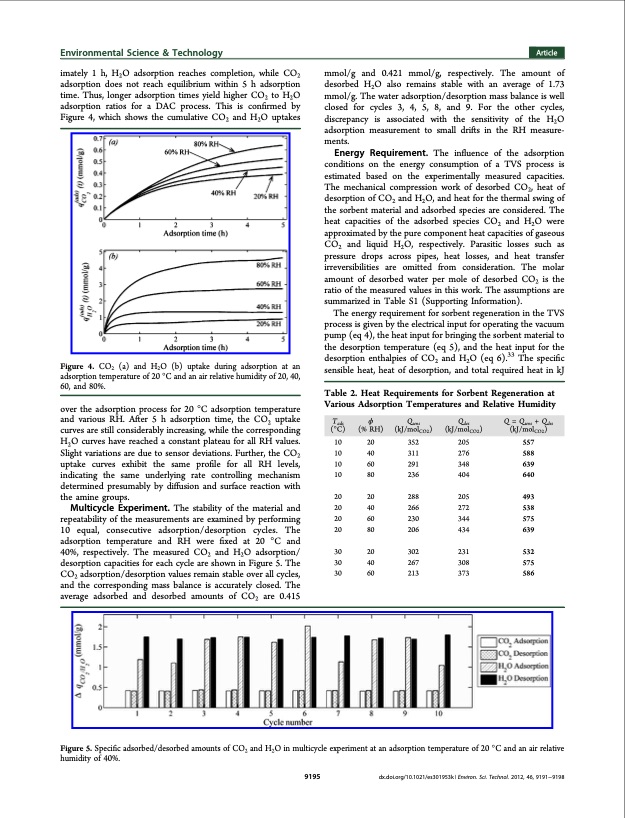
Environmental Science & Technology Article imately 1 h, H2O adsorption reaches completion, while CO2 adsorption does not reach equilibrium within 5 h adsorption time. Thus, longer adsorption times yield higher CO2 to H2O adsorption ratios for a DAC process. This is confirmed by Figure 4, which shows the cumulative CO2 and H2O uptakes Figure 4. CO2 (a) and H2O (b) uptake during adsorption at an adsorption temperature of 20 °C and an air relative humidity of 20, 40, 60, and 80%. over the adsorption process for 20 °C adsorption temperature and various RH. After 5 h adsorption time, the CO2 uptake curves are still considerably increasing, while the corresponding H2O curves have reached a constant plateau for all RH values. Slight variations are due to sensor deviations. Further, the CO2 uptake curves exhibit the same profile for all RH levels, indicating the same underlying rate controlling mechanism determined presumably by diffusion and surface reaction with the amine groups. Multicycle Experiment. The stability of the material and repeatability of the measurements are examined by performing 10 equal, consecutive adsorption/desorption cycles. The adsorption temperature and RH were fixed at 20 °C and 40%, respectively. The measured CO2 and H2O adsorption/ desorption capacities for each cycle are shown in Figure 5. The CO2 adsorption/desorption values remain stable over all cycles, and the corresponding mass balance is accurately closed. The average adsorbed and desorbed amounts of CO2 are 0.415 mmol/g and 0.421 mmol/g, respectively. The amount of desorbed H2O also remains stable with an average of 1.73 mmol/g. The water adsorption/desorption mass balance is well closed for cycles 3, 4, 5, 8, and 9. For the other cycles, discrepancy is associated with the sensitivity of the H2O adsorption measurement to small drifts in the RH measure- ments. Energy Requirement. The influence of the adsorption conditions on the energy consumption of a TVS process is estimated based on the experimentally measured capacities. The mechanical compression work of desorbed CO2, heat of desorption of CO2 and H2O, and heat for the thermal swing of the sorbent material and adsorbed species are considered. The heat capacities of the adsorbed species CO2 and H2O were approximated by the pure component heat capacities of gaseous CO2 and liquid H2O, respectively. Parasitic losses such as pressure drops across pipes, heat losses, and heat transfer irreversibilities are omitted from consideration. The molar amount of desorbed water per mole of desorbed CO2 is the ratio of the measured values in this work. The assumptions are summarized in Table S1 (Supporting Information). The energy requirement for sorbent regeneration in the TVS process is given by the electrical input for operating the vacuum pump (eq 4), the heat input for bringing the sorbent material to the desorption temperature (eq 5), and the heat input for the desorption enthalpies of CO2 and H2O (eq 6).33 The specific sensible heat, heat of desorption, and total required heat in kJ Table 2. Heat Requirements for Sorbent Regeneration at Various Adsorption Temperatures and Relative Humidity 10 20 352 205 557 10 40 311 276 588 10 60 291 348 639 10 80 236 404 640 20 20 288 205 493 20 40 266 272 538 20 60 230 344 575 20 80 206 434 639 30 20 302 231 532 30 40 267 308 575 30 60 213 373 586 Tads φ Qsens Qdes Q = Qsens + Qdes (°C) (% RH) (kJ/molCO2) (kJ/molCO2) (kJ/molCO2) Figure 5. Specific adsorbed/desorbed amounts of CO2 and H2O in multicycle experiment at an adsorption temperature of 20 °C and an air relative humidity of 40%. 9195 dx.doi.org/10.1021/es301953k | Environ. Sci. Technol. 2012, 46, 9191−9198PDF Image | Concurrent Separation of CO2 and H2O from Air PSA
PDF Search Title:
Concurrent Separation of CO2 and H2O from Air PSAOriginal File Name Searched:
Separation-of-CO2-and-H2O-PSApdf.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |