
PDF Publication Title:
Text from PDF Page: 036
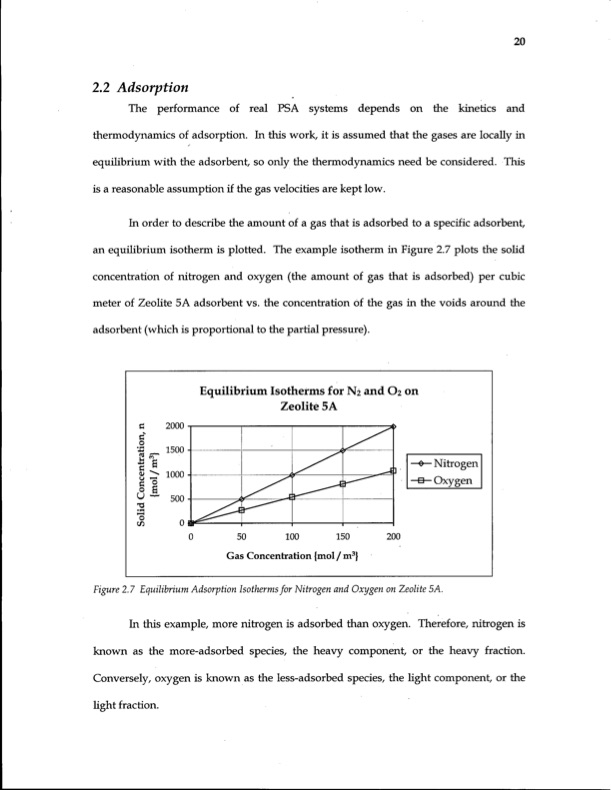
2.2 Adsorption The performance of real PSA systems depends on the kinetics and thermodynamics of adsorption. In this work, it is assumed that the gases are locally in equilibrium with the adsorbent, so only the thermodynamics need be considered. This is a reasonable assumption if the gas velocities are kept low. In order to describe the amount of a gas that is adsorbed to a specific adsorbent, an equilibrium isotherm is plotted. The example isotherm in Figure 2.7 plots the solid concentration of nitrogen and oxygen (the amount of gas that is adsorbed) per cubic meter of Zeolite 5A adsorbent vs. the concentration of the gas in the voids around the adsorbent (which is proportional to the partial pressure). Equilibrium Isotherms for N2 and O2 on Zeolite 5A -e— Nitrogen •a—Oxygen Gas Concentration {mol / m3} Figure 2.7 Equilibrium Adsorption Isothermsfor Nitrogen and Oxygen onZeolite 5A. In this example, more nitrogen is adsorbed than oxygen. Therefore, nitrogen is known as the more-adsorbed species, the heavy component, or the heavy fraction. Conversely, oxygen is known as the less-adsorbed species, the light component, or the light fraction. 20PDF Image | Energy Efficiency of Gas Separation Pressure Swing Adsorption
PDF Search Title:
Energy Efficiency of Gas Separation Pressure Swing AdsorptionOriginal File Name Searched:
ubc_1997-0009.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |