
PDF Publication Title:
Text from PDF Page: 020
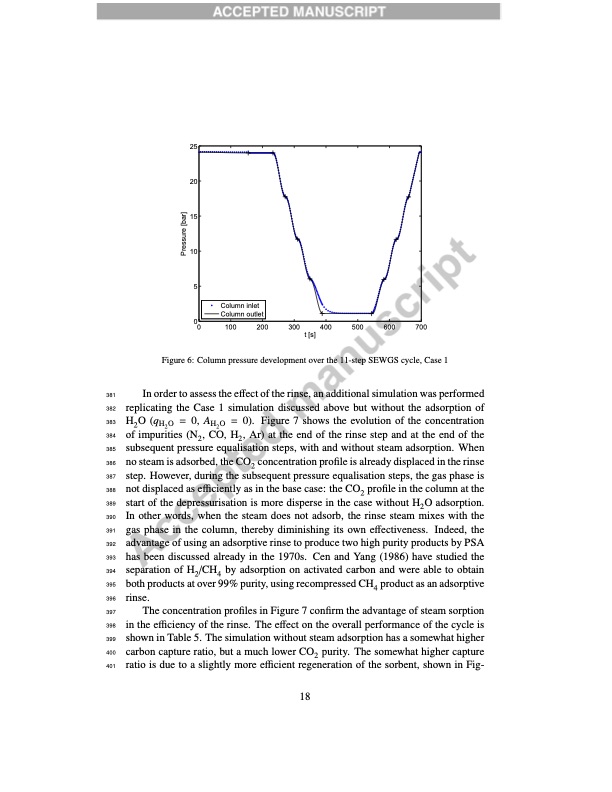
25 20 15 10 5 00 100 200 300 Figure 6: Column pressure development over the 11-step SEWGS cycle, Case 1 381 In order to assess the effect of the rinse, an additional simulation was performed 382 replicating the Case 1 simulation discussed above but without the adsorption of 383 H2 O (qH2 O = 0, AH2 O = 0). Figure 7 shows the evolution of the concentration 384 of impurities (N2, CO, H2, Ar) at the end of the rinse step and at the end of the 385 subsequent pressure equalisation steps, with and without steam adsorption. When 386 no steam is adsorbed, the CO2 concentration profile is already displaced in the rinse 387 step. However, during the subsequent pressure equalisation steps, the gas phase is 388 not displaced as efficiently as in the base case: the CO2 profile in the column at the 389 start of the depressurisation is more disperse in the case without H2O adsorption. 390 In other words, when the steam does not adsorb, the rinse steam mixes with the 391 gas phase in the column, thereby diminishing its own effectiveness. Indeed, the 392 advantage of using an adsorptive rinse to produce two high purity products by PSA 393 has been discussed already in the 1970s. Cen and Yang (1986) have studied the 394 separation of H2/CH4 by adsorption on activated carbon and were able to obtain 395 both products at over 99% purity, using recompressed CH4 product as an adsorptive 396 rinse. 397 The concentration profiles in Figure 7 confirm the advantage of steam sorption 398 in the efficiency of the rinse. The effect on the overall performance of the cycle is 399 shown in Table 5. The simulation without steam adsorption has a somewhat higher 400 carbon capture ratio, but a much lower CO2 purity. The somewhat higher capture 401 ratio is due to a slightly more efficient regeneration of the sorbent, shown in Fig- 18 Column inlet Column outlet t [s] 400 500 600 700 Pressure [bar]PDF Image | High-temperature pressure swing adsorption cycle design for sorption
PDF Search Title:
High-temperature pressure swing adsorption cycle design for sorptionOriginal File Name Searched:
high-temperature-pressure-swing-adsorption.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |