
PDF Publication Title:
Text from PDF Page: 027
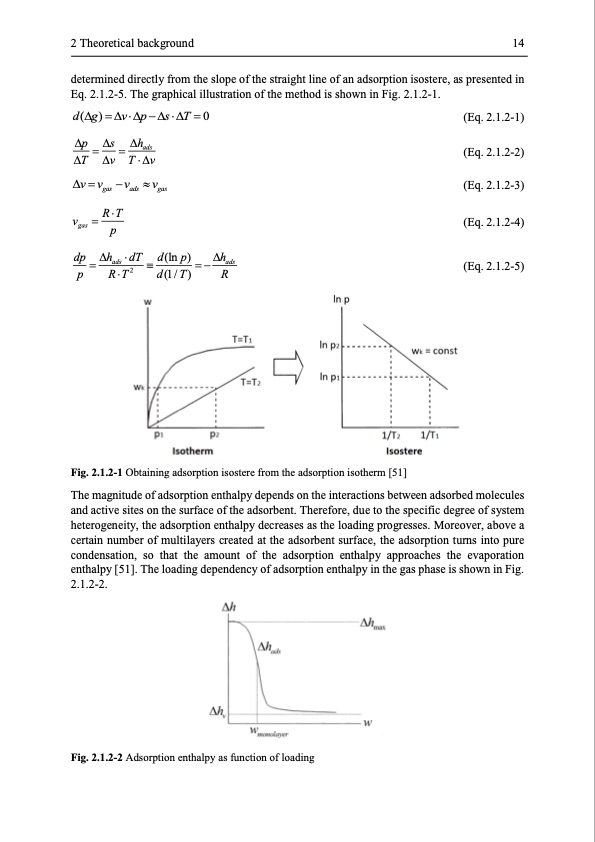
2 Theoretical background 14 determined directly from the slope of the straight line of an adsorption isostere, as presented in Eq. 2.1.2-5. The graphical illustration of the method is shown in Fig. 2.1.2-1. d(g)=vp−sT =0 p = s = hads T v Tv v=vgas −vads vgas vgas =RT p dp = hads dT d (ln p) = − hads p RT2 d(1/T) R (Eq. 2.1.2-1) (Eq. 2.1.2-2) (Eq. 2.1.2-3) (Eq. 2.1.2-4) (Eq. 2.1.2-5) Fig. 2.1.2-1 Obtaining adsorption isostere from the adsorption isotherm [51] The magnitude of adsorption enthalpy depends on the interactions between adsorbed molecules and active sites on the surface of the adsorbent. Therefore, due to the specific degree of system heterogeneity, the adsorption enthalpy decreases as the loading progresses. Moreover, above a certain number of multilayers created at the adsorbent surface, the adsorption turns into pure condensation, so that the amount of the adsorption enthalpy approaches the evaporation enthalpy [51]. The loading dependency of adsorption enthalpy in the gas phase is shown in Fig. 2.1.2-2. Fig. 2.1.2-2 Adsorption enthalpy as function of loadingPDF Image | Modelling and Simulation of Twin-Bed Pressure Swing Adsorption Plants
PDF Search Title:
Modelling and Simulation of Twin-Bed Pressure Swing Adsorption PlantsOriginal File Name Searched:
dissertation_marcinek.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |