
PDF Publication Title:
Text from PDF Page: 050
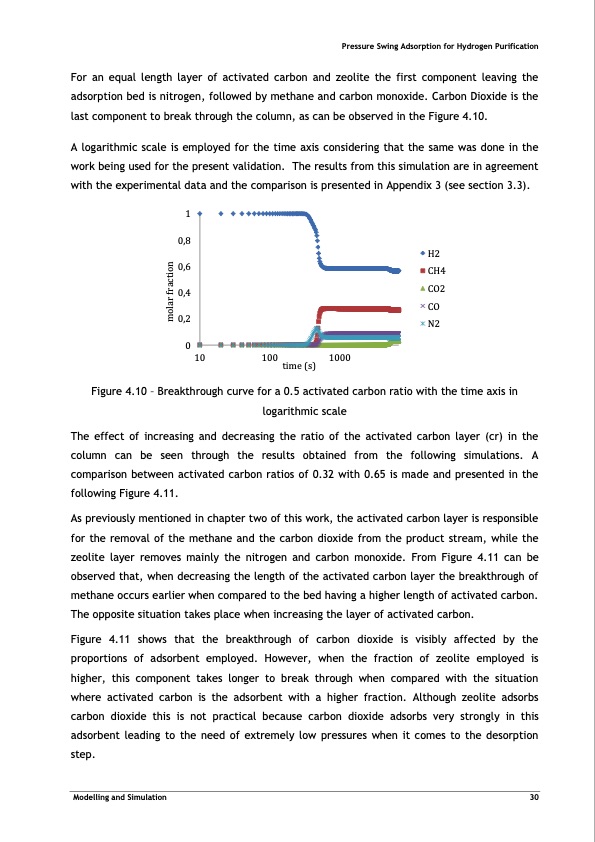
For an equal length layer of activated carbon and zeolite the first component leaving the adsorption bed is nitrogen, followed by methane and carbon monoxide. Carbon Dioxide is the last component to break through the column, as can be observed in the Figure 4.10. A logarithmic scale is employed for the time axis considering that the same was done in the work being used for the present validation. The results from this simulation are in agreement with the experimental data and the comparison is presented in Appendix 3 (see section 3.3). Figure 4.10 – Breakthrough curve for a 0.5 activated carbon ratio with the time axis in logarithmic scale The effect of increasing and decreasing the ratio of the activated carbon layer (cr) in the column can be seen through the results obtained from the following simulations. A comparison between activated carbon ratios of 0.32 with 0.65 is made and presented in the following Figure 4.11. As previously mentioned in chapter two of this work, the activated carbon layer is responsible for the removal of the methane and the carbon dioxide from the product stream, while the zeolite layer removes mainly the nitrogen and carbon monoxide. From Figure 4.11 can be observed that, when decreasing the length of the activated carbon layer the breakthrough of methane occurs earlier when compared to the bed having a higher length of activated carbon. The opposite situation takes place when increasing the layer of activated carbon. Figure 4.11 shows that the breakthrough of carbon dioxide is visibly affected by the proportions of adsorbent employed. However, when the fraction of zeolite employed is higher, this component takes longer to break through when compared with the situation where activated carbon is the adsorbent with a higher fraction. Although zeolite adsorbs carbon dioxide this is not practical because carbon dioxide adsorbs very strongly in this adsorbent leading to the need of extremely low pressures when it comes to the desorption step. Pressure Swing Adsorption for Hydrogen Purification 1 0,8 0,6 0,4 0,2 0 H2 CH4 CO2 CO N2 10 100 time (s) 1000 Modelling and Simulation 30 molar fractionPDF Image | PRESSURE SWING ADSORPTION FOR THE PURIFICATION OF HYDROGEN
PDF Search Title:
PRESSURE SWING ADSORPTION FOR THE PURIFICATION OF HYDROGENOriginal File Name Searched:
32541.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |