
PDF Publication Title:
Text from PDF Page: 006
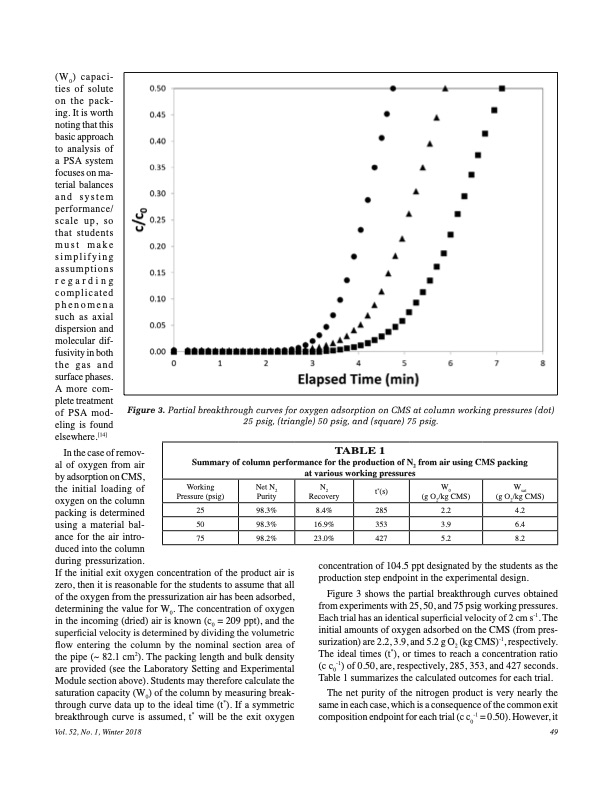
(W0) capaci- ties of solute on the pack- ing. It is worth noting that this basic approach to analysis of a PSA system focuses on ma- terial balances and system performance/ scale up, so that students must make simplifying assumptions regarding complicated phenomena such as axial dispersion and molecular dif- fusivity in both the gas and surface phases. A more com- plete treatment of PSA mod- eling is found elsewhere.[14] Figure 3. Partial breakthrough curves for oxygen adsorption on CMS at column working pressures (dot) Figure 3: Partial breakthrough curves for oxygen adsorption on CMS at column In the case of remov- al of oxygen from air by adsorption on CMS, the initial loading of oxygen on the column packing is determined using a material bal- ance for the air intro- duced into the column during pressurization. If the initial exit oxygen concentration of the product air is zero, then it is reasonable for the students to assume that all of the oxygen from the pressurization air has been adsorbed, determining the value for W0. The concentration of oxygen in the incoming (dried) air is known (c0 = 209 ppt), and the superficial velocity is determined by dividing the volumetric flow entering the column by the nominal section area of the pipe (~ 82.1 cm2). The packing length and bulk density are provided (see the Laboratory Setting and Experimental Module section above). Students may therefore calculate the saturation capacity (W0) of the column by measuring break- through curve data up to the ideal time (t*). If a symmetric breakthrough curve is assumed, t* will be the exit oxygen Vol. 52, No. 1, Winter 2018 25 psig, (triangle) 50 psig, and (square) 75 psig. working pressures (●) 25 psig, (▲) 50 psig, and (■) 75 psig. TABLE 1 Summary of column performance for the production of N2 from air using CMS packing at various working pressures Working Pressure (psig) Net N N t (s) W W 22* 0 sat Purity Recovery (g O2/kg CMS) (g O2/kg CMS) 98.3% 8.4% 285 2.2 4.2 25 50 98.3% 16.9% 353 3.9 6.4 75 98.2% 23.0% 427 5.2 8.2 concentration of 104.5 ppt designated by the students as the production step endpoint in the experimental design. Figure 3 shows the partial breakthrough curves obtained from experiments with 25, 50, and 75 psig working pressures. Each trial has an identical superficial velocity of 2 cm s-1. The initial amounts of oxygen adsorbed on the CMS (from pres- surization) are 2.2, 3.9, and 5.2 g O (kg CMS)-1, respectively. 2 The ideal times (t*), or times to reach a concentration ratio (c c0-1) of 0.50, are, respectively, 285, 353, and 427 seconds. Table 1 summarizes the calculated outcomes for each trial. The net purity of the nitrogen product is very nearly the same in each case, which is a consequence of the common exit composition endpoint for each trial (c c0-1 = 0.50). However, it 3 49PDF Image | PRESSURE SWING ADSORPTION IN THE UNIT OPERATIONS
PDF Search Title:
PRESSURE SWING ADSORPTION IN THE UNIT OPERATIONSOriginal File Name Searched:
PSA-lab.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |