
PDF Publication Title:
Text from PDF Page: 008
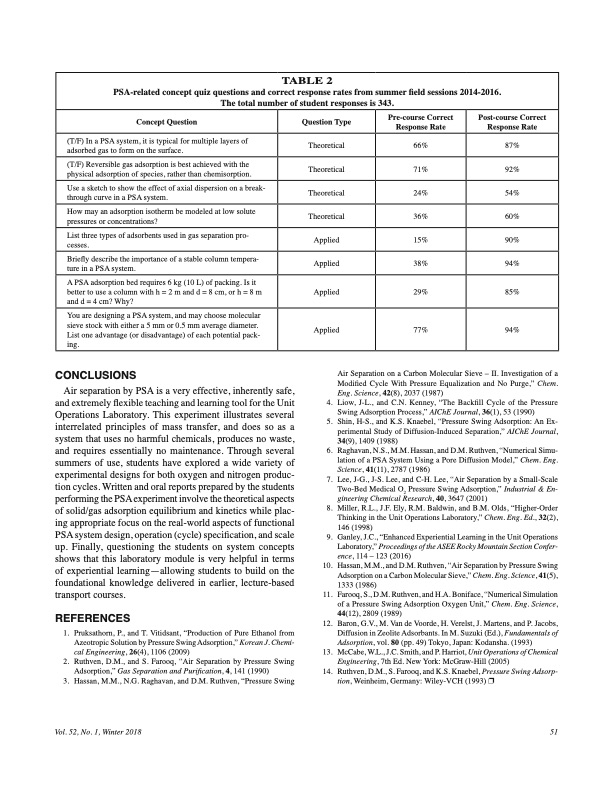
TABLE 2 PSA-related concept quiz questions and correct response rates from summer field sessions 2014-2016. The total number of student responses is 343. Concept Question (T/F) In a PSA system, it is typical for multiple layers of adsorbed gas to form on the surface. (T/F) Reversible gas adsorption is best achieved with the physical adsorption of species, rather than chemisorption. Use a sketch to show the effect of axial dispersion on a break- through curve in a PSA system. How may an adsorption isotherm be modeled at low solute pressures or concentrations? List three types of adsorbents used in gas separation pro- cesses. Briefly describe the importance of a stable column tempera- ture in a PSA system. A PSA adsorption bed requires 6 kg (10 L) of packing. Is it better to use a column with h = 2 m and d = 8 cm, or h = 8 m and d = 4 cm? Why? CONCLUSIONS Air separation by PSA is a very effective, inherently safe, and extremely flexible teaching and learning tool for the Unit Operations Laboratory. This experiment illustrates several interrelated principles of mass transfer, and does so as a system that uses no harmful chemicals, produces no waste, and requires essentially no maintenance. Through several summers of use, students have explored a wide variety of experimental designs for both oxygen and nitrogen produc- tion cycles. Written and oral reports prepared by the students performing the PSA experiment involve the theoretical aspects of solid/gas adsorption equilibrium and kinetics while plac- ing appropriate focus on the real-world aspects of functional PSA system design, operation (cycle) specification, and scale up. Finally, questioning the students on system concepts shows that this laboratory module is very helpful in terms of experiential learning—allowing students to build on the foundational knowledge delivered in earlier, lecture-based transport courses. REFERENCES Question Type Theoretical Theoretical Theoretical Theoretical Applied Applied Applied Pre-course Correct Response Rate 66% 71% 24% 36% 15% 38% 29% Post-course Correct Response Rate 87% 92% 54% 60% 90% 94% 85% You are designing a PSA system, and may choose molecular sieve stock with either a 5 mm or 0.5 mm average diameter. List one advantage (or disadvantage) of each potential pack- ing. Applied 77% 94% 1. Pruksathorn, P., and T. Vitidsant, “Production of Pure Ethanol from Azeotropic Solution by Pressure Swing Adsorption,” Korean J. Chemi- cal Engineering, 26(4), 1106 (2009) 12. Baron, G.V., M. Van de Voorde, H. Verelst, J. Martens, and P. Jacobs, Diffusion in Zeolite Adsorbants. In M. Suzuki (Ed.), Fundamentals of Adsorption, vol. 80 (pp. 49) Tokyo, Japan: Kodansha. (1993) 2. Ruthven, D.M., and S. Farooq, “Air Separation by Pressure Swing Adsorption,” Gas Separation and Purification, 4, 141 (1990) 13. McCabe, W.L., J.C. Smith, and P. Harriot, Unit Operations of Chemical Engineering, 7th Ed. New York: McGraw-Hill (2005) 3. Hassan, M.M., N.G. Raghavan, and D.M. Ruthven, “Pressure Swing 14. Ruthven, D.M., S. Farooq, and K.S. Knaebel, Pressure Swing Adsorp- tion, Weinheim, Germany: Wiley-VCH (1993) p Vol. 52, No. 1, Winter 2018 51 Air Separation on a Carbon Molecular Sieve – II. Investigation of a Modified Cycle With Pressure Equalization and No Purge,” Chem. Eng. Science, 42(8), 2037 (1987) 4. Liow, J-L., and C.N. Kenney, “The Backfill Cycle of the Pressure Swing Adsorption Process,” AIChE Journal, 36(1), 53 (1990) 5. Shin, H-S., and K.S. Knaebel, “Pressure Swing Adsorption: An Ex- perimental Study of Diffusion-Induced Separation,” AIChE Journal, 34(9), 1409 (1988) 6. Raghavan, N.S., M.M. Hassan, and D.M. Ruthven, “Numerical Simu- lation of a PSA System Using a Pore Diffusion Model,” Chem. Eng. Science, 41(11), 2787 (1986) 7. Lee, J-G., J-S. Lee, and C-H. Lee, “Air Separation by a Small-Scale Two-Bed Medical O2 Pressure Swing Adsorption,” Industrial & En- gineering Chemical Research, 40, 3647 (2001) 8. Miller, R.L., J.F. Ely, R.M. Baldwin, and B.M. Olds, “Higher-Order Thinking in the Unit Operations Laboratory,” Chem. Eng. Ed., 32(2), 146 (1998) 9. Ganley, J.C., “Enhanced Experiential Learning in the Unit Operations Laboratory,” Proceedings of the ASEE Rocky Mountain Section Confer- ence, 114 – 123 (2016) 10. Hassan, M.M., and D.M. Ruthven, “Air Separation by Pressure Swing Adsorption on a Carbon Molecular Sieve,” Chem. Eng. Science, 41(5), 1333 (1986) 11. Farooq, S., D.M. Ruthven, and H.A. Boniface, “Numerical Simulation of a Pressure Swing Adsorption Oxygen Unit,” Chem. Eng. Science, 44(12), 2809 (1989)PDF Image | PRESSURE SWING ADSORPTION IN THE UNIT OPERATIONS
PDF Search Title:
PRESSURE SWING ADSORPTION IN THE UNIT OPERATIONSOriginal File Name Searched:
PSA-lab.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |