
PDF Publication Title:
Text from PDF Page: 069
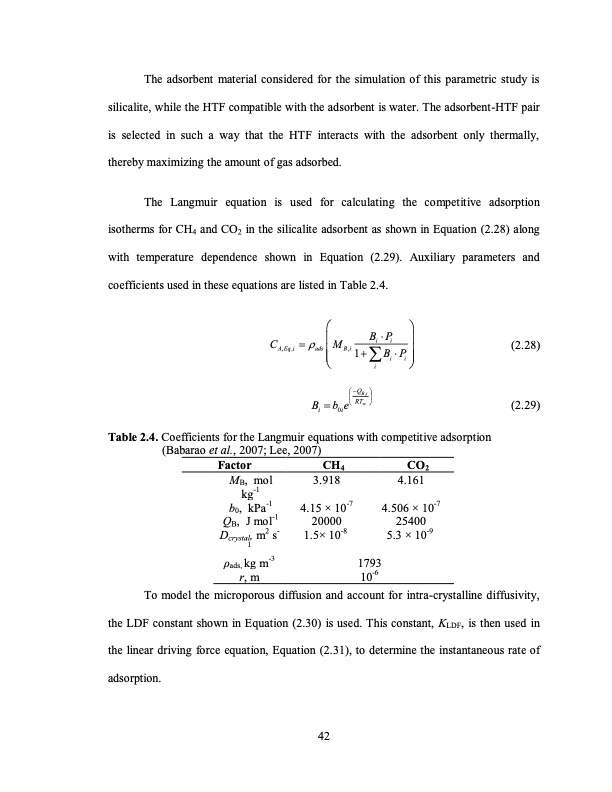
The adsorbent material considered for the simulation of this parametric study is silicalite, while the HTF compatible with the adsorbent is water. The adsorbent-HTF pair is selected in such a way that the HTF interacts with the adsorbent only thermally, thereby maximizing the amount of gas adsorbed. The Langmuir equation is used for calculating the competitive adsorption isotherms for CH4 and CO2 in the silicalite adsorbent as shown in Equation (2.28) along with temperature dependence shown in Equation (2.29). Auxiliary parameters and coefficients used in these equations are listed in Table 2.4. BP CM ii (2.28) (2.29) A,Eq,i ads B,i1BP Table 2.4. Coefficients for the Langmuir equations with competitive adsorption (Babarao et al., 2007; Lee, 2007) ii i BbeRT i 0i QB,i w Factor MB, mol kg-1 b0, kPa-1 QB, J mol-1 Dcrystal, m2 s- 1 ρads, kg m-3 r, m CH4 3.918 4.15 × 10-7 20000 1.5× 10-8 CO2 4.161 4.506 × 10-7 25400 5.3 × 10-9 1793 10-6 To model the microporous diffusion and account for intra-crystalline diffusivity, the LDF constant shown in Equation (2.30) is used. This constant, KLDF, is then used in the linear driving force equation, Equation (2.31), to determine the instantaneous rate of adsorption. 42PDF Image | TEMPERATURE SWING ADSORPTION PROCESSES FOR GAS SEPARATION
PDF Search Title:
TEMPERATURE SWING ADSORPTION PROCESSES FOR GAS SEPARATIONOriginal File Name Searched:
PAHINKAR-DISSERTATION-2016.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |