
PDF Publication Title:
Text from PDF Page: 209
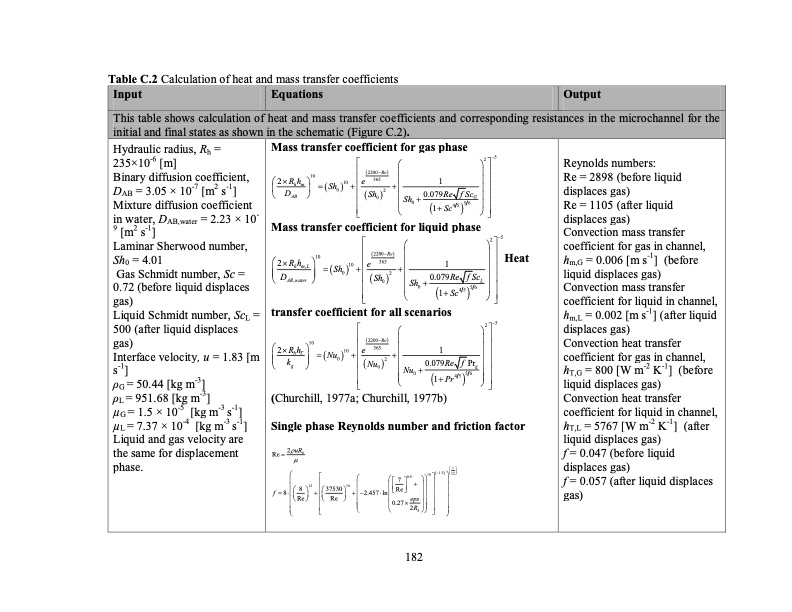
Table C.2 Calculation of heat and mass transfer coefficients Input Equations Output This table shows calculation of heat and mass transfer coefficients and corresponding resistances in the microchannel for the initial and final states as shown in the schematic (Figure C.2). Hydraulic radius, Rh = 235×10-6 [m] Binary diffusion coefficient, DAB = 3.05 × 10-7 [m2 s-1] Mixture diffusion coefficient Mass transfer coefficient for gas phase Reynolds numbers: Re = 2898 (before liquid displaces gas) Re = 1105 (after liquid 2 5 2200Re 365 10 2Rh 10 e 1 hmSh0 2 DAB Sh0 Sh0.079RefScG 0 4556 1Sc Mass transfer coefficient for liquid phase 2Rh 10 365 hm,L 10e 1 2 5 2200Re Heat Sh 02 DAB,water Sh0 Sh 0.079Re fScL transfer coefficient for all scenarios 2200Re 10 365 0 4556 1Sc 2 5 2Rh 10e 1 h T Nu k 0 g Nu2 0.079RefPr 0Nu0 g 56 1Pr45 in water, DAB,water = 2.23 × 10- 9 [m2 s-1] Laminar Sherwood number, Sh0 = 4.01 Gas Schmidt number, Sc = 0.72 (before liquid displaces gas) Liquid Schmidt number, ScL = 500 (after liquid displaces gas) Interface velocity, u = 1.83 [m s-1] ρG = 50.44 [kg m-3] displaces gas) Convection mass transfer coefficient for gas in channel, hm,G = 0.006 [m s-1] (before liquid displaces gas) Convection mass transfer coefficient for liquid in channel, hm,L = 0.002 [m s-1] (after liquid displaces gas) Convection heat transfer coefficient for gas in channel, hT,G = 800 [W m-2 K-1] (before liquid displaces gas) ρL = 951.68 [kg m-3] μG = 1.5 × 10-5 [kg m-3 s-1] μL = 7.37 × 10-4 [kg m-3 s-1] Liquid and gas velocity are the same for displacement phase. (Churchill, 1977a; Churchill, 1977b) Single phase Reynolds number and friction factor Re 2uRh 1 0 . 9 1 6 1 . 5 1 2 12 16 7 f 8 8 37530 2.457lnRe Re Re 0.27eps 2R h Convection heat transfer coefficient for liquid in channel, hT,L = 5767 [W m-2 K-1] (after liquid displaces gas) f = 0.047 (before liquid displaces gas) f = 0.057 (after liquid displaces gas) 182PDF Image | TEMPERATURE SWING ADSORPTION PROCESSES FOR GAS SEPARATION
PDF Search Title:
TEMPERATURE SWING ADSORPTION PROCESSES FOR GAS SEPARATIONOriginal File Name Searched:
PAHINKAR-DISSERTATION-2016.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |