
PDF Publication Title:
Text from PDF Page: 016
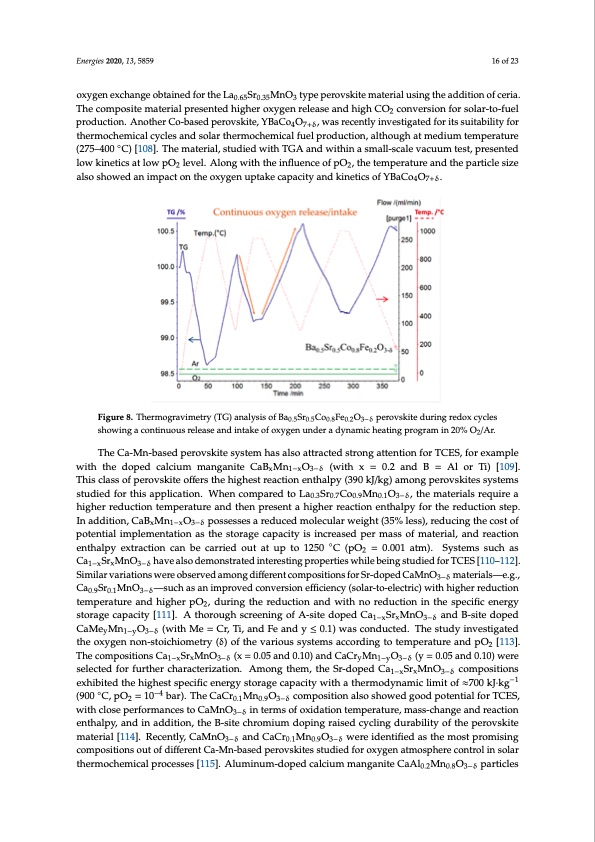
Energies 2020, 13, 5859 16 of 23 oxygen exchange obtained for the La0.65Sr0.35MnO3 type perovskite material using the addition of ceria. The composite material presented higher oxygen release and high CO2 conversion for solar-to-fuel production. Another Co-based perovskite, YBaCo4O7+δ, was recently investigated for its suitability for thermochemical cycles and solar thermochemical fuel production, although at medium temperature (275–400 ◦C) [108]. The material, studied with TGA and within a small-scale vacuum test, presented low kinetics at low pO2 level. Along with the influence of pO2, the temperature and the particle size also showed an impact on the oxygen uptake capacity and kinetics of YBaCo4O7+δ. Energies 2020, 13, x FOR PEER REVIEW 16 of 24 Figure 8. Thermogravimetry (TG) analysis of Ba0.5Sr0.5Co0.8Fe0.2O3−δ perovskite during redox cycles Figure 8. Thermogravimetry (TG) analysis of Ba0.5Sr0.5Co0.8Fe0.2O3−δ perovskite during redox cycles showing a continuous release and intake of oxygen under a dynamic heating program in 20% O2/Ar. showing a continuous release and intake of oxygen under a dynamic heating program in 20% O2/Ar. The BaySr1−yCoO3−δ system was also studied, along with LaxSr1−x (Mn, Fe, Co)O3−δ, by Gokon et al. [104]. The study concluded on the suitability of Ba0.3Sr0.7CoO3−δ and Ba0.7Sr0.3CoO3−δ for TCES above The Ca-Mn-based perovskite system has also attracted strong attention for TCES, for example 600 °C in air stream. It was noted that no direct correlation was observed between the oxygen storage with the dopecdapaccaitlycaiundmthemteandnegncaynoiftetheChaeaBt sMtornage caOpacity fo(rwthietshe sxyste=ms.0F.2or caonmdparBison=, itAisl or Ti) [109]. x 1−x 3−δ mentioned that the charging/discharging capacity of Ba0.3Sr0.7CoO3−δ is higher than that of Fe-doped This class of perovskite offers the highest reaction enthalpy (390 kJ/kg) among perovskites systems manganese oxides, which have been shown to be a promising system for TCES. The LaxSr1−x(Mn, Fe, rials require a studied for thisCoa)pOpliscyastteimonw.asWstuhdeiend fcuortmherpwaritehdfotcuos LonatheSLraxSrCoCoyMMn1−n O(LSCM,)tahned mLax e 3−δ xCoyFe1−yO3−δ (LSCF) series [105,106]. TGA and structural investigation revealed that the systems with higher reduction temperature and then present a higher reaction enthalpy for the reduction step. low La content presented the highest redox activity, with an optimum reached for x = 0.3, while the In addition, CaB Mn O possesses a reduced molecular weight (35% less), reducing the cost of pexrovski1te−sxado3p−tδed a cubic structure, or tetragonal structure for LSCM. Higher La content led to a potential implementation as the storage capacity is increased per mass of material, and reaction higher distortion in the perovskite structure, related to a decrease in redox activity. Among all the systems studied, the LSCM3791 composition presented the highest gravimetric energy density (250 enthalpy extraction can be carried out at up to 1250 ◦C (pO2 = 0.001 atm). Systems such as kJ/kg-ABO3). Very recently, dual-phase La0.65Sr0.35MnO3−xCeO2 composites (with x = 0, 5, 10, 20, 50, 2 Ca SrMnOandha10v0e%)alwseoredienvmesotingastterdaftoerdoxiyngtenrexscthinangeparnodpCeOrtiespsliwttinhgi,levibaethinergmoscthuedmiecadlrfeodroxTCES[110–112]. 1−x x 3−δ reactions, for the purpose of fuel production [107]. This work demonstrated the enhancement in Similar variations were observed among different compositions for Sr-doped CaMnO3−δ materials—e.g., oxygen exchange obtained for the La0.65Sr0.35MnO3 type perovskite material using the addition of ceria. 2 cro-ntove-reslioenctforrics)olwar-ittoh-fuheligherreduction Ca Sr MnO The—cosmupcohsitaesmatneriaml prersoevntedhcigohnervoexrysgieonnreeleffiaseciaenndchyigh(sCoOla 0.9 0.1 3−δ production. Another Co-based perovskite, YBaCo4O7+δ, was recently investigated for its suitability temperature and higher pO2, during the reduction and with no reduction in the specific energy for thermochemical cycles and solar thermochemical fuel production, although at medium storage capacity [111]. A thorough screening of A-site doped Ca Sr MnO and B-site doped temperature (275–400 °C) [108]. The material, studied with TGA and wit1h−inxa smx all-scale3−vδacuum CaMe Mn O (with Me = Cr, Ti, and Fe and y ≤ 0.1) was conducted. The study investigated y 1−y 3−δ test, presented low kinetics at low pO2 level. Along with the influence of pO2, the temperature and the particle size also showed an impact on the oxygen uptake capacity and kinetics of YBaCo4O7+δ. the oxygen non-stoichiometry (δ) of the various systems according to temperature and pO2 [113]. The Ca-Mn-based perovskite system has also attracted strong attention for TCES, for example ThecompositionwsithCtahedopSerdMcalcniuOmman(gxan=ite0C.a0B51(w0i)thaxn=d0.C2anCdrB=MAnlorTiO)[109].(Tyhis=cl0as.s05ofand0.10)were 1−x x 3−δ . y 1−y 3−δ 1− xMand xO0 3− δ perovskite offers the highest reaction enthalpy (390 kJ/kg) among perovskites systems studied for selected for further characterization. Among them, the Sr-doped Ca1−xSrxMnO3−δ compositions this application. When compared to La0.3Sr0.7Co0.9Mn0.1O3−δ, the materials require a higher reduction −1 exhibited the hitegmhpesrattusrpe eacnidfitcheennperregseynt satohrigahgere rceacptioancietnythwalpiythfoar tthhe erremducotidonynstaepm. Iinc alidmdititono, f ≈700 kJ·kg ◦ 0.3 0.7 0.9 0.1δ 3−δ CaB−x4Mn1−xO3−δ possesses a reduced molecular weight (35% less), reducing the cost of potential C, pO2 = 10 bar). The CaCr0.1Mn0.9O3−δ composition also showed good potential for TCES, (900 with close performances to CaMnO3−δ in terms of oxidation temperature, mass-change and reaction enthalpy, and in addition, the B-site chromium doping raised cycling durability of the perovskite material [114]. Recently, CaMnO3−δ and CaCr0.1Mn0.9O3−δ were identified as the most promising compositions out of different Ca-Mn-based perovskites studied for oxygen atmosphere control in solar thermochemical processes [115]. Aluminum-doped calcium manganite CaAl0.2Mn0.8O3−δ particles 1−x - yO3− aStr1PDF Image | Hi Temp Thermochemical Energy Storage via Solid Gas Reactions
PDF Search Title:
Hi Temp Thermochemical Energy Storage via Solid Gas ReactionsOriginal File Name Searched:
energies-13-05859.pdfDIY PDF Search: Google It | Yahoo | Bing
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
IT XR Project Redstone NFT Available for Sale: NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Be part of the future with this NFT. Can be bought and sold but only one design NFT exists. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Turbine IT XR Project Redstone Design: NFT for sale... NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Includes all rights to this turbine design, including license for Fluid Handling Block I and II for the turbine assembly and housing. The NFT includes the blueprints (cad/cam), revenue streams, and all future development of the IT XR Project Redstone... More Info
Infinity Turbine ROT Radial Outflow Turbine 24 Design and Worldwide Rights: NFT for sale... NFT for the ROT 24 energy turbine. Be part of the future with this NFT. This design can be bought and sold but only one design NFT exists. You may manufacture the unit, or get the revenues from its sale from Infinity Turbine. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Supercritical CO2 10 Liter Extractor Design and Worldwide Rights: The Infinity Supercritical 10L CO2 extractor is for botanical oil extraction, which is rich in terpenes and can produce shelf ready full spectrum oil. With over 5 years of development, this industry leader mature extractor machine has been sold since 2015 and is part of many profitable businesses. The process can also be used for electrowinning, e-waste recycling, and lithium battery recycling, gold mining electronic wastes, precious metals. CO2 can also be used in a reverse fuel cell with nafion to make a gas-to-liquids fuel, such as methanol, ethanol and butanol or ethylene. Supercritical CO2 has also been used for treating nafion to make it more effective catalyst. This NFT is for the purchase of worldwide rights which includes the design. More Info
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
Infinity Turbine Products: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. May pay by Bitcoin or other Crypto. Products Page... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |