
PDF Publication Title:
Text from PDF Page: 003
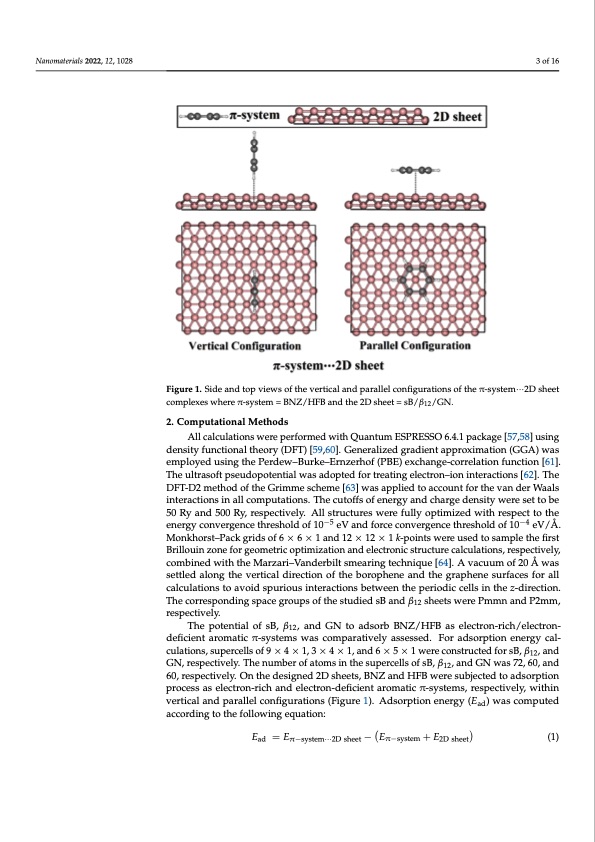
Nanomaterials 2022, 12, 1028 3 of 16 Figure 1. Side and top views of the vertical and parallel configurations of the π-system···2D sheet complexes where π-system = BNZ/HFB and the 2D sheet = sB/β12/GN. 2. Computational Methods All calculations were performed with Quantum ESPRESSO 6.4.1 package [57,58] using density functional theory (DFT) [59,60]. Generalized gradient approximation (GGA) was employed using the Perdew–Burke–Ernzerhof (PBE) exchange-correlation function [61]. The ultrasoft pseudopotential was adopted for treating electron–ion interactions [62]. The DFT-D2 method of the Grimme scheme [63] was applied to account for the van der Waals interactions in all computations. The cutoffs of energy and charge density were set to be 50 Ry and 500 Ry, respectively. All structures were fully optimized with respect to the energy convergence threshold of 10−5 eV and force convergence threshold of 10−4 eV/Å. Monkhorst–Pack grids of 6 × 6 × 1 and 12 × 12 × 1 k-points were used to sample the first Brillouin zone for geometric optimization and electronic structure calculations, respectively, combined with the Marzari–Vanderbilt smearing technique [64]. A vacuum of 20 Å was settled along the vertical direction of the borophene and the graphene surfaces for all calculations to avoid spurious interactions between the periodic cells in the z-direction. The corresponding space groups of the studied sB and β12 sheets were Pmmn and P2mm, respectively. The potential of sB, β12, and GN to adsorb BNZ/HFB as electron-rich/electron- deficient aromatic π-systems was comparatively assessed. For adsorption energy cal- culations, supercells of 9 × 4 × 1, 3 × 4 × 1, and 6 × 5 × 1 were constructed for sB, β12, and GN, respectively. The number of atoms in the supercells of sB, β12, and GN was 72, 60, and 60, respectively. On the designed 2D sheets, BNZ and HFB were subjected to adsorption process as electron-rich and electron-deficient aromatic π-systems, respectively, within vertical and parallel configurations (Figure 1). Adsorption energy (Ead) was computed according to the following equation: Ead = Eπ−system···2D sheet − Eπ−system + E2D sheet (1)PDF Image | Borophene and Pristine Graphene 2D Sheets
PDF Search Title:
Borophene and Pristine Graphene 2D SheetsOriginal File Name Searched:
nanomaterials-12-01028-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |