
PDF Publication Title:
Text from PDF Page: 011
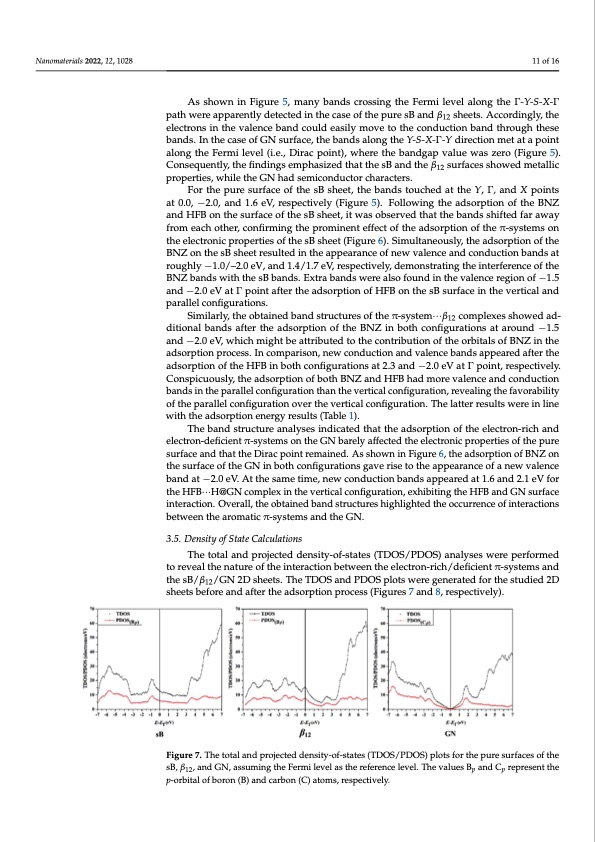
Nanomaterials 2022, 12, 1028 11 of 16 As shown in Figure 5, many bands crossing the Fermi level along the ð-Y-S-X-ð path were apparently detected in the case of the pure sB and β12 sheets. Accordingly, the electrons in the valence band could easily move to the conduction band through these bands. In the case of GN surface, the bands along the Y-S-X-ð-Y direction met at a point along the Fermi level (i.e., Dirac point), where the bandgap value was zero (Figure 5). Consequently, the findings emphasized that the sB and the β12 surfaces showed metallic properties, while the GN had semiconductor characters. For the pure surface of the sB sheet, the bands touched at the Y, ð, and X points at 0.0, −2.0, and 1.6 eV, respectively (Figure 5). Following the adsorption of the BNZ and HFB on the surface of the sB sheet, it was observed that the bands shifted far away from each other, confirming the prominent effect of the adsorption of the π-systems on the electronic properties of the sB sheet (Figure 6). Simultaneously, the adsorption of the BNZ on the sB sheet resulted in the appearance of new valence and conduction bands at roughly −1.0/–2.0 eV, and 1.4/1.7 eV, respectively, demonstrating the interference of the BNZ bands with the sB bands. Extra bands were also found in the valence region of −1.5 and −2.0 eV at ð point after the adsorption of HFB on the sB surface in the vertical and parallel configurations. Similarly, the obtained band structures of the π-system···β12 complexes showed ad- ditional bands after the adsorption of the BNZ in both configurations at around −1.5 and −2.0 eV, which might be attributed to the contribution of the orbitals of BNZ in the adsorption process. In comparison, new conduction and valence bands appeared after the adsorption of the HFB in both configurations at 2.3 and −2.0 eV at ð point, respectively. Conspicuously, the adsorption of both BNZ and HFB had more valence and conduction bands in the parallel configuration than the vertical configuration, revealing the favorability of the parallel configuration over the vertical configuration. The latter results were in line with the adsorption energy results (Table 1). The band structure analyses indicated that the adsorption of the electron-rich and electron-deficient π-systems on the GN barely affected the electronic properties of the pure surface and that the Dirac point remained. As shown in Figure 6, the adsorption of BNZ on the surface of the GN in both configurations gave rise to the appearance of a new valence band at −2.0 eV. At the same time, new conduction bands appeared at 1.6 and 2.1 eV for the HFB···H@GN complex in the vertical configuration, exhibiting the HFB and GN surface interaction. Overall, the obtained band structures highlighted the occurrence of interactions between the aromatic π-systems and the GN. 3.5. Density of State Calculations The total and projected density-of-states (TDOS/PDOS) analyses were performed to reveal the nature of the interaction between the electron-rich/deficient π-systems and the sB/β12/GN 2D sheets. The TDOS and PDOS plots were generated for the studied 2D sheets before and after the adsorption process (Figures 7 and 8, respectively). Figure 7. The total and projected density-of-states (TDOS/PDOS) plots for the pure surfaces of the sB, β12, and GN, assuming the Fermi level as the reference level. The values Bp and Cp represent the p-orbital of boron (B) and carbon (C) atoms, respectively.PDF Image | Borophene and Pristine Graphene 2D Sheets
PDF Search Title:
Borophene and Pristine Graphene 2D SheetsOriginal File Name Searched:
nanomaterials-12-01028-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |