
PDF Publication Title:
Text from PDF Page: 013
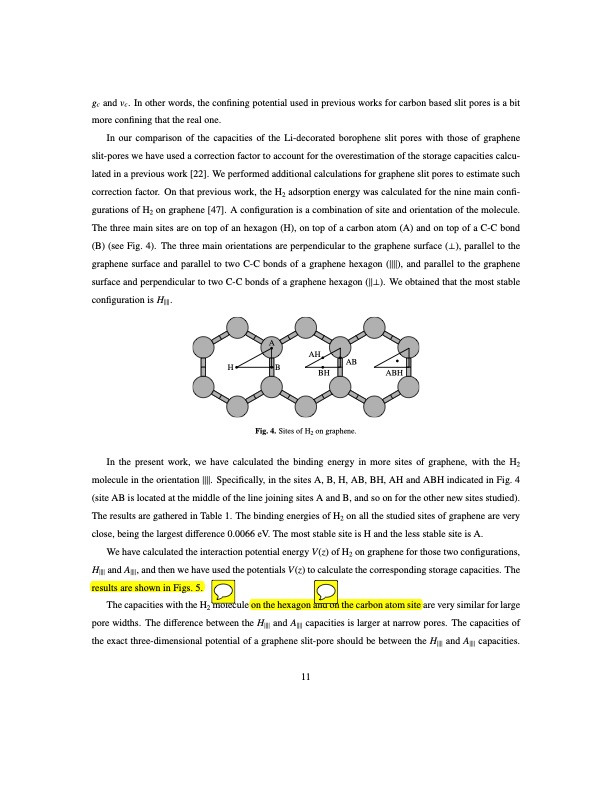
gc and vc. In other words, the confining potential used in previous works for carbon based slit pores is a bit more confining that the real one. In our comparison of the capacities of the Li-decorated borophene slit pores with those of graphene slit-pores we have used a correction factor to account for the overestimation of the storage capacities calcu- lated in a previous work [22]. We performed additional calculations for graphene slit pores to estimate such correction factor. On that previous work, the H2 adsorption energy was calculated for the nine main confi- gurations of H2 on graphene [47]. A configuration is a combination of site and orientation of the molecule. The three main sites are on top of an hexagon (H), on top of a carbon atom (A) and on top of a C-C bond (B) (see Fig. 4). The three main orientations are perpendicular to the graphene surface (⊥), parallel to the graphene surface and parallel to two C-C bonds of a graphene hexagon (∥∥), and parallel to the graphene surface and perpendicular to two C-C bonds of a graphene hexagon (∥⊥). We obtained that the most stable configuration is H∥∥. A AH HB AB BH ABH Fig. 4. Sites of H2 on graphene. In the present work, we have calculated the binding energy in more sites of graphene, with the H2 molecule in the orientation ∥∥. Specifically, in the sites A, B, H, AB, BH, AH and ABH indicated in Fig. 4 (site AB is located at the middle of the line joining sites A and B, and so on for the other new sites studied). The results are gathered in Table 1. The binding energies of H2 on all the studied sites of graphene are very close, being the largest difference 0.0066 eV. The most stable site is H and the less stable site is A. We have calculated the interaction potential energy V(z) of H2 on graphene for those two configurations, H∥∥ and A∥∥, and then we have used the potentials V(z) to calculate the corresponding storage capacities. The results are shown in Figs. 5. The capacities with the H2 molecule on the hexagon and on the carbon atom site are very similar for large pore widths. The difference between the H∥∥ and A∥∥ capacities is larger at narrow pores. The capacities of the exact three-dimensional potential of a graphene slit-pore should be between the H∥∥ and A∥∥ capacities. 11PDF Image | Hydrogen storage capacity of Li-decorated borophene
PDF Search Title:
Hydrogen storage capacity of Li-decorated boropheneOriginal File Name Searched:
APSUSC-D-20-15170.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |