
PDF Publication Title:
Text from PDF Page: 016
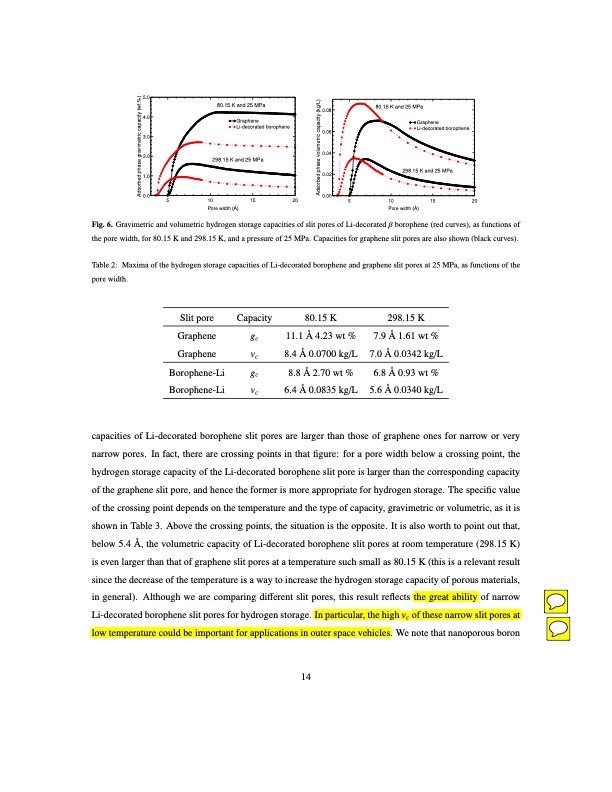
5.0 4.0 3.0 2.0 1.0 0.0 5 10 15 20 Pore width (Å) 80.15 K and 25 MPa Graphene Li-decorated borophene 298.15 K and 25 MPa 0.08 0.06 0.04 0.02 0.00 5 10 15 20 Pore width (Å) 80.15 K and 25 MPa Graphene Li-decorated borophene 298.15 K and 25 MPa Fig. 6. Gravimetric and volumetric hydrogen storage capacities of slit pores of Li-decorated β borophene (red curves), as functions of the pore width, for 80.15 K and 298.15 K, and a pressure of 25 MPa. Capacities for graphene slit pores are also shown (black curves). Table2: MaximaofthehydrogenstoragecapacitiesofLi-decoratedboropheneandgrapheneslitporesat25MPa,asfunctionsofthe pore width. Slit pore Graphene Graphene Borophene-Li Borophene-Li Capacity 80.15 K gc 11.1 Å 4.23 wt % vc 8.4 Å 0.0700 kg/L gc 8.8 Å 2.70 wt % vc 6.4 Å 0.0835 kg/L 298.15 K 7.9 Å 1.61 wt % 7.0 Å 0.0342 kg/L 6.8 Å 0.93 wt % 5.6 Å 0.0340 kg/L capacities of Li-decorated borophene slit pores are larger than those of graphene ones for narrow or very narrow pores. In fact, there are crossing points in that figure: for a pore width below a crossing point, the hydrogen storage capacity of the Li-decorated borophene slit pore is larger than the corresponding capacity of the graphene slit pore, and hence the former is more appropriate for hydrogen storage. The specific value of the crossing point depends on the temperature and the type of capacity, gravimetric or volumetric, as it is shown in Table 3. Above the crossing points, the situation is the opposite. It is also worth to point out that, below 5.4 Å, the volumetric capacity of Li-decorated borophene slit pores at room temperature (298.15 K) is even larger than that of graphene slit pores at a temperature such small as 80.15 K (this is a relevant result since the decrease of the temperature is a way to increase the hydrogen storage capacity of porous materials, in general). Although we are comparing different slit pores, this result reflects the great ability of narrow Li-decorated borophene slit pores for hydrogen storage. In particular, the high vc of these narrow slit pores at low temperature could be important for applications in outer space vehicles. We note that nanoporous boron 14 Adsorbed phase gravimetric capacity (wt %) Adsorbed phase volumetric capacity (kg/L)PDF Image | Hydrogen storage capacity of Li-decorated borophene
PDF Search Title:
Hydrogen storage capacity of Li-decorated boropheneOriginal File Name Searched:
APSUSC-D-20-15170.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |