
PDF Publication Title:
Text from PDF Page: 018
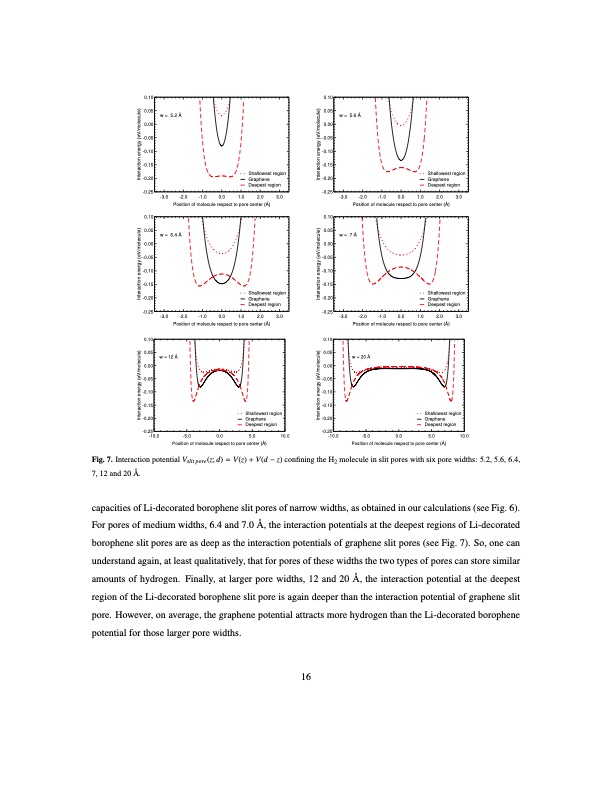
0.10 0.05 0.00 -0.05 -0.10 -0.15 -0.20 -0.25 0.10 0.05 0.00 -0.05 -0.10 -0.15 -0.20 -0.25 0.10 0.05 0.00 -0.05 -0.10 -0.15 -0.20 -0.25 -10.0 w = 5.2 Å 0.10 0.05 0.00 -0.05 -0.10 -0.15 Shallowest region Graphene -0.20 Deepest region w = 5.6 Å Interaction energy (eV/molecule) Interaction energy (eV/molecule) Interaction energy (eV/molecule) Interaction energy (eV/molecule) Interaction energy (eV/molecule) Interaction energy (eV/molecule) -3.0 -2.0 Position of molecule respect to pore center (Å) 3.0 -0.25 0.10 0.05 0.00 -0.05 -0.10 -0.15 -3.0 -2.0 -1.0 0.0 1.0 2.0 3.0 Position of molecule respect to pore center (Å) w = 6.4 Å w= 7 Å -3.0 -2.0 Position of molecule respect to pore center (Å) 3.0 -0.25 0.10 0.05 0.00 -0.05 -0.10 -0.15 -3.0 -2.0 -1.0 0.0 1.0 2.0 3.0 Position of molecule respect to pore center (Å) -1.0 0.0 1.0 2.0 -1.0 0.0 1.0 2.0 w = 12 Å -5.0 Position of molecule respect to pore center (Å) w = 20 Å 0.0 -0.25 -10.0 0.0 5.0 10.0 Shallowest region Graphene -0.20 Deepest region Shallowest region Graphene Deepest region Shallowest region Graphene -0.20 Deepest region Shallowest region Graphene Deepest region 5.0 10.0 -5.0 Position of molecule respect to pore center (Å) Fig. 7. Interaction potential Vslit pore (z; d) = V (z) + V (d − z) confining the H2 molecule in slit pores with six pore widths: 5.2, 5.6, 6.4, 7, 12 and 20 Å. capacities of Li-decorated borophene slit pores of narrow widths, as obtained in our calculations (see Fig. 6). For pores of medium widths, 6.4 and 7.0 Å, the interaction potentials at the deepest regions of Li-decorated borophene slit pores are as deep as the interaction potentials of graphene slit pores (see Fig. 7). So, one can understand again, at least qualitatively, that for pores of these widths the two types of pores can store similar amounts of hydrogen. Finally, at larger pore widths, 12 and 20 Å, the interaction potential at the deepest region of the Li-decorated borophene slit pore is again deeper than the interaction potential of graphene slit pore. However, on average, the graphene potential attracts more hydrogen than the Li-decorated borophene potential for those larger pore widths. 16 Shallowest region Graphene Deepest regionPDF Image | Hydrogen storage capacity of Li-decorated borophene
PDF Search Title:
Hydrogen storage capacity of Li-decorated boropheneOriginal File Name Searched:
APSUSC-D-20-15170.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |