
PDF Publication Title:
Text from PDF Page: 003
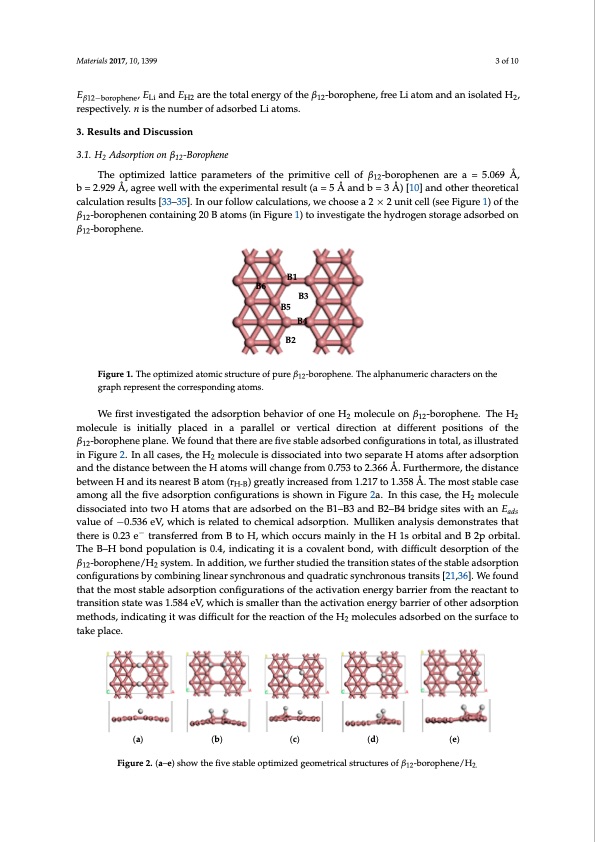
Materials 2017, 10, 1399 3 of 10 Eβ12−borophene, ELi and EH2 are the total energy of the β12-borophene, free Li atom and an isolated H2, respectively. n is the number of adsorbed Li atoms. Materials 2017, 10, 1399 3 of 10 3 of 10 The optimized lattice parameters of the primitive cell of β -borophenen are a = 5.069 Å, The optimized lattice parameters of the primitive cell of β12-borophe12nen are a = 5.069 Å, b = 2.929 Å, 3. Results and Discussion 3. Results and Discussion 3.1. H Adsorption on β -Borophene M2aterials 2017, 10, 1399 12 3.1. H2 Adsorption on β12-Borophene 3. Results and Discussion b = 2.a9g2r9eeÅw, aegllrweeithwtehlel wexiptheritmhenetxapl reersiumlte(nata=l5reÅsualntd(ab = 53 Å)a[1n0d] ban=d 3otÅhe)r[1th0e]oarentdicaolthcaelrcutlhaetiornetical calcu3lra.e1ts.iuoHlnt2sAre[d3s3uo–rl3pts5ti]o[.3nI3on–n3oβ5u1]2.r-BIfnoorlolpouhwrenfeocallocuwlactiaolncsu,lawtieoncsh,owosecaho2os×e2a2un×it2cuelnli(tsceellF(isgeuereFi1g)uroef1t)heofthe β12-borophenen containing 20 B atoms (in Figure 1) to investigate the hydrogen storage adsorbed on β12-borophenen containing 20 B atoms (in Figure 1) to investigate the hydrogen storage adsorbed on The optimized lattice parameters of the primitive cell of β12-borophenen are a = 5.069 Å, b = 2.929 Å, β12-borophene. β12-borophene. agree well with the experimental result (a = 5 Å and b = 3 Å) [10] and other theoretical calculation results [33–35]. In our follow calculations, we choose a 2 × 2 unit cell (see Figure 1) of the β12-borophenen containing 20 B atoms (in Figure 1) to investigate the hydrogen storage adsorbed on β12-borophene. B1 B6 B3 B5 B1 B6 B3 B5 B4 B2 B4 Figure 1. The optimized atomic structure of pure β12-borophene. The alphanumeric characters on the Figure 1. The optimized atomic structure of pure β12-borophene. The alphanumeric characters on the graph represent the corresponding atoms. B2 graph represent the corresponding atoms. We first investigated the adsorption behavior of one H2 molecule on β12-borophene. The H2 We fiFrigsutrien1v.eTshteigopatiemdiztehdeataodmsicosrtpruticotunreboefhpauvreioβ1 molecule is initially placed in a parallel or vertical direction at different positions of the β12-borophene graph represent the corresponding atoms. molecule is initially placed in a parallel or vertical direction at different positions of the plane. We found that there are five stable adsorbed configurations in total, as illustrated in Figure 2. β12-boInroapllhceasnes,ptlhaenHe.2 mWoelefoculnedistdhiasstotchieartedairnetofivtweostsaebplaeratdesHorabtoemd scoafntfiergaudrsaotriopntiosninantodttahl,eadsisitlalunscterated We first investigated the adsorption behavior of one H2 molecule on β12-borophene. The H2 in Figbuertwe e2e.nInthaelHl catsoemss, twhiellHchanmgoelfercoumle0.i7s53dtioss2o.3c6ia6tÅed. Fiunrttohetrwmorse,pthaeradtiestHancaetobmetws eaefnteHr adndsoirtsption 2 molecule is initially placed in a parallel or vertical direction at different positions of the β12-borophene nearest B atom (rH-B) greatly increased from 1.217 to 1.358 Å. The most stable case among all the five and the distance between the H atoms will change from 0.753 to 2.366 Å. Furthermore, the distance plane. We found that there are five stable adsorbed configurations in total, as illustrated in Figure 2. adsorption configurations is shown in Figure 2a. In this case, the H2 molecule dissociated into two H between H and its nearest B atom (rH-B) greatly increased from 1.217 to 1.358 Å. The most stable case In all cases, the H2 molecule is dissociated into two separate H atoms after adsorption and the distance atoms that are adsorbed on the B1–B3 and B2–B4 bridge sites with an value of −0.536 eV, which among all the five adsorption configurations is shown in Figure 2a. In this case, the H molecule between the H atoms will change from 0.753 to 2.366 Å. Furthermore, the distance between H a2nd its is related to chemical adsorption. Mulliken analysis demonstrates that there is 0.23 e− transferred from dissonceiatresdt Binattomtw(orH-HB) garteoamtlys itnhcarteasreedafrdosmor1b.2e1d7 oton1t.h35e8BÅ1.–TBh3e amnodstBs2ta–bBle4cbarseidagmeosnigteasllwthiethfivaen Eads B to H, which occurs mainly in the H 1s orbital and B 2p orbital. The B–H bond population is 0.4, valueadosfo−rp0ti.o5n36coenVfi,gwurhaitciohnsisisreshlaotwednitnoFcihguermei2ca.lInadthsiosrcpatsieo,nth.eMHu2mllioklecnulaendailsysosicsiadtedminotnosttwraoteHsthat indicating it is a covalent bond, with difficult desorption of the β12-borophene/H2 system. In addition, atoms that are adsorbed on the B1–B3 and B2–B4 bridge sites with an value of −0.536 eV, which there is 0.23 e− transferred from B to H, which occurs mainly in theH 1s orbital and B 2p orbital. we further studied the transition states of the stable adsorption configurations by combining linear is related to chemical adsorption. Mulliken analysis demonstrates that there is 0.23 e− transferred from The B–H bond population is 0.4, indicating it is a covalent bond, with difficult desorption of the synchronous and quadratic synchronous transits [21,36]. We found that the most stable adsorption B to H, which occurs mainly in the H 1s orbital and B 2p orbital. The B–H bond population is 0.4, β -bocoronpfihguenraet/ioHnssoyfsttheema.cItnivatdiodnitieonne,rgwyebfaurrtihererfrsotmudtiheedrtehaectaranntstiotitornansstiatitoensosftattheewstaasb1le.5a8d4seoVr,ption 12 2 indicating it is a covalent bond, with difficult desorption of the β12-borophene/H2 system. In addition, configwuhriacthioinssmbyalcleormthbaininthgelaincteiavratsiyoncehnreorngoyubsaarrniedrqoufaodthreartiacdssyonrcphtiroonnmouesthtoradns,sintsd[i2ca1t,i3n6g].itWweafsound we further studied the transition states of the stable adsorption configurations by combining linear difficult for the reaction of the H2 molecules adsorbed on the surface to take place. that the most stable adsorption configurations of the activation energy barrier from the reactant to synchronous and quadratic synchronous transits [21,36]. We found that the most stable adsorption transition state was 1.584 eV, which is smaller than the activation energy barrier of other adsorption configurations of the activation energy barrier from the reactant to transition state was 1.584 eV, methods, indicating it was difficult for the reaction of the H molecules adsorbed on the surface to which is smaller than the activation energy barrier of other a2dsorption methods, indicating it was take pdliaffciceu. lt for the reaction of the H2 molecules adsorbed on the surface to take place. (a) (b) (c) (d) (e) Figure 2. (a–e) show the five stable optimized geometrical structures of β12-borophene/H2. (a) (b) (c) (d) (e) Figure 2. (a–e) show the five stable optimized geometrical structures of β12-borophene/H2. Figure 2. (a–e) show the five stable optimized geometrical structures of β12-borophene/H2. r 2-boofropnhenHe. Thmeoalpehcaunluemoenricβcha-rbaoctreorspohnetnhe. The H 2 12 2PDF Image | Li-Decorated Borophene as Potentia for Hydrogen Storage
PDF Search Title:
Li-Decorated Borophene as Potentia for Hydrogen StorageOriginal File Name Searched:
materials-10-01399.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |