
PDF Publication Title:
Text from PDF Page: 007
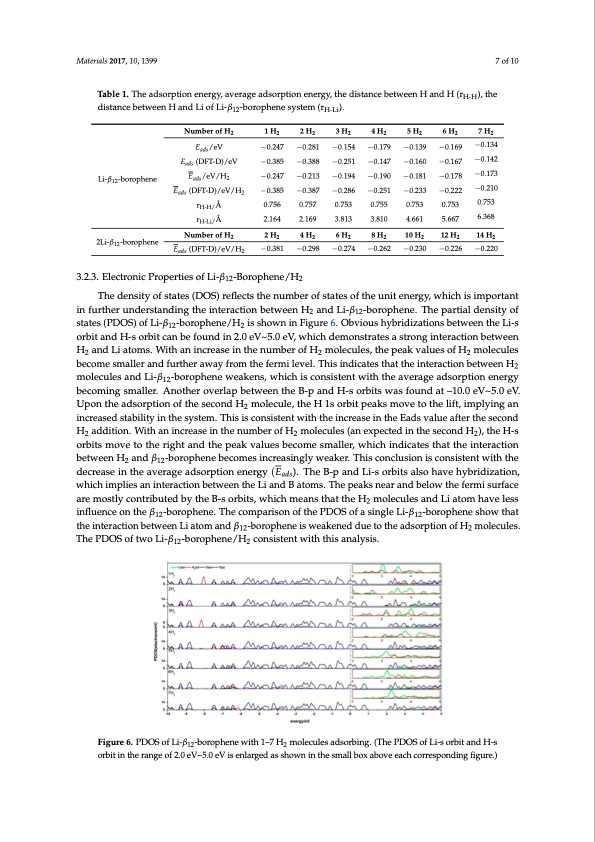
Materials 2017, 10, 1399 7 of 10 Table 1. The adsorption energy, average adsorption energy, the distance between H and H (rH-H), the distance between H and Li of Li-β12-borophene system (rH-Li). Number of H2 Materials 2017, 10, 1399 Eads/eV 1 H2 −0.247 2 H2 −0.281 3 H2 −0.154 Li-β12-borophene Li-β12- borophene 2Li-β12-borophene /eV/H2 −0.213 2 H2 −0.387 −0.194 4 H2 6 H2 −0.286 −0.190 8 H2 −0.251 −0.181 −0.178 −0.173 4 H2 0.753 −0.2470.756−0.2810.757−0.1540.753−0.1790.755−0.1390.753−0.1690.753−0.134 −0.142 Table 1. The adsorption energy, average adsorption energy, the distance between H and H (rH-H), −0.385 −0.385 −0.387 −0.286 −0.251 −0.233 −0.222 −0.388 2 1 H 2 2 H 2 3 H 2 4 H 2 5 H 2 6 H 2 7 H 2 Eads (DFT-D)/eV thedistancebetweEeandsH/eaVn/dHL2iofLi-β12-−bo0.r2o4p7hene−s0y.2st1e3m(r−H-L0i).1.94 −0.190 −0.181 −0.178 −0.173 −0.210 E (DFT-D)/eV/H N ua dms b e r o f H 2 r/eV Å H-H/ (DFT-D)/eV −0.385 −0.247 −0.385 −0.388 −0.251 −0.147 −0.160 −0.167 −0.142 r Å H-Li/ 2.164 2.169 3.813 3.810 4.661 5.667 6.368 −0.251 −0.167 5 H2 6 H2 7 H2 −0.169 7 o−f 100.134 −0.179 −0.147 −0.139 −0.160 Nu(DmFbTe-rDo)f H2 rH-H/Å rH-Li/Å 10 H2 12 H2 14 H2 −0.233 −0.222 −0.210 /eV/H2 Eads (DFT-D)/eV/H2 −0.381 −0.298 −0.274 −0.262 −0.230 −0.226 −0.220 3.2.3. Electronic Properties of Li-β 2Li-β12- Number of H2 0.756 0.757 2.164 2.169 0.753 3.813 6 H2 −0.274 0.755 3.810 8 H2 −0.262 0.753 4.661 10 H2 −0.230 0.753 5.667 12 H2 −0.226 0.753 6.368 14 H2 −0.220 -Borophene/H 2 H2 4 H2 2 12 The density of states (DOS) reflects the number of states of the unit energy, which is important borophene (DFT-D) /eV/H2 −0.381 −0.298 in further understanding the interaction between H2 and Li-β12-borophene. The partial density of 3.2.3. Electronic Properties of Li-β12-Borophene/H2 states (PDOS) of Li-β12-borophene/H2 is shown in Figure 6. Obvious hybridizations between the Li-s orbit and H-s orbit can be found in 2.0 eV~5.0 eV, which demonstrates a strong interaction between The density of states (DOS) reflects the number of states of the unit energy, which is important H andiLnifautrothmers.uWndietrhstandingcrtehaeseinitenratchteionubmetwbeernofHH2andmLoil-eβc12u-bleorso,pthenpe.eTahkevpaalrutieaslodfenHsitymooflecules 222 states (PDOS) of Li-β12-borophene/H2 is shown in Figure 6. Obvious hybridizations between the Li-s become smaller and further away from the fermi level. This indicates that the interaction between H2 orbit and H-s orbit can be found in 2.0 eV~5.0 eV, which demonstrates a strong interaction between molecules and Li-β12-borophene weakens, which is consistent with the average adsorption energy H2 and Li atoms. With an increase in the number of H2 molecules, the peak values of H2 molecules becoming smaller. Another overlap between the B-p and H-s orbits was found at –10.0 eV~5.0 eV. become smaller and further away from the fermi level. This indicates that the interaction between H2 Upon the adsorption of the second H molecule, the H 1s orbit peaks move to the lift, implying an molecules and Li-β12-borophene we2akens, which is consistent with the average adsorption energy increasebdecsotambinilgitysminalltehre. Asynsotehmer.oTvehrilsapisbceotwnseiesntetnhet wB-ipthanthdeHi-nscorrebaistsewinasthfoeuEnaddast v–1a0lu.0eeaVf~t5e.r0tehVe. second Upon the adsorption of the second H2 molecule, the H 1s orbit peaks move to the lift, implying an H2 addition. With an increase in the number of H2 molecules (an expected in the second H2), the H-s increased stability in the system. This is consistent with the increase in the Eads value after the second orbits move to the right and the peak values become smaller, which indicates that the interaction H2 addition. With an increase in the number of H2 molecules (an expected in the second H2), the H-s between H2 and β12-borophene becomes increasingly weaker. This conclusion is consistent with the orbits move to the right and the peak values become smaller, which indicates that the interaction decrease in the average adsorption energy (Eads). The B-p and Li-s orbits also have hybridization, between H2 and β12-borophene becomes increasingly weaker. This conclusion is consistent with the which implies an interaction between the Li and B atoms. The peaks near and below the fermi surface decrease in the average adsorption energy (). The B-p and Li-s orbits also have hybridization, aremoswtlhyichoinmtprilbieustaendinbteyratchteionB-bsetowrebeintst,hweLhiiacnhdmBaetaonmss.thThaetptheaekHsneamroanledcbueleoswatnhedfeLrimaitsoumrfahceaveless 2 are mostly contributed by the B-s orbits, which means that the H2 molecules and Li atom have less influence on the β12-borophene. The comparison of the PDOS of a single Li-β12-borophene show that influence on the β12-borophene. The comparison of the PDOS of a single Li-β12-borophene show that the interaction between Li atom and β12-borophene is weakened due to the adsorption of H2 molecules. the interaction between Li atom and β12-borophene is weakened due to the adsorption of H2 The PDOS of two Li-β12-borophene/H2 consistent with this analysis. molecules. The PDOS of two Li-β12-borophene/H2 consistent with this analysis. Figure 6. PDOS of Li-β12-borophene with 1–7 H2 molecules adsorbing. (The PDOS of Li-s orbit and Figure 6. PDOS of Li-β12-borophene with 1–7 H2 molecules adsorbing. (The PDOS of Li-s orbit and H-s H-s orbit in the range of 2.0 eV~5.0 eV is enlarged as shown in the small box above each corresponding orbit in the range of 2.0 eV~5.0 eV is enlarged as shown in the small box above each corresponding figure.) figure.)PDF Image | Li-Decorated Borophene as Potentia for Hydrogen Storage
PDF Search Title:
Li-Decorated Borophene as Potentia for Hydrogen StorageOriginal File Name Searched:
materials-10-01399.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |