
PDF Publication Title:
Text from PDF Page: 004
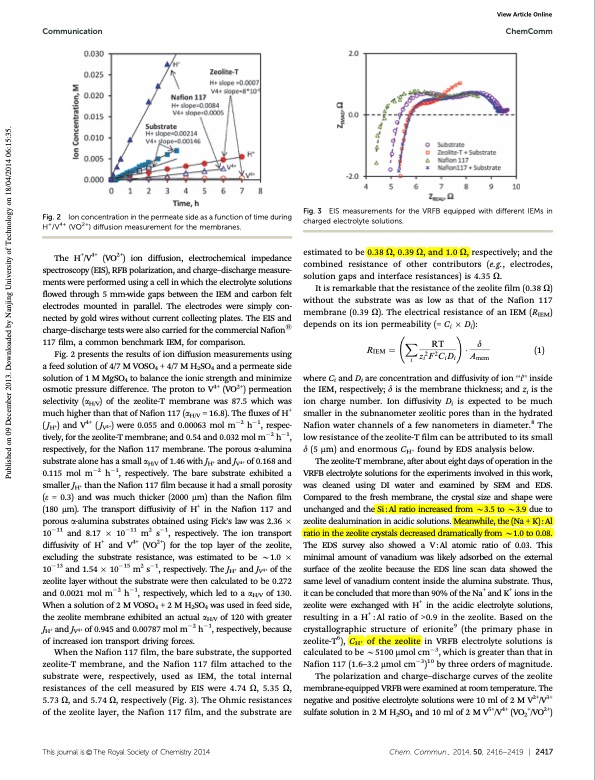
Communication ChemComm Fig. 3 EIS measurements for the VRFB equipped with different IEMs in charged electrolyte solutions. estimated to be 0.38 O, 0.39 O, and 1.0 O, respectively; and the combined resistance of other contributors (e.g., electrodes, solution gaps and interface resistances) is 4.35 O. It is remarkable that the resistance of the zeolite film (0.38 O) without the substrate was as low as that of the Nafion 117 membrane (0.39 O). The electrical resistance of an IEM (RIEM) depends on its ion permeability (= Ci Di): X RT ! d RIEM 1⁄4 z2F2CD A (1) ii ii mem where Ci and Di are concentration and diffusivity of ion ‘‘i’’ inside the IEM, respectively; d is the membrane thickness; and zi is the ion charge number. Ion diffusivity Di is expected to be much smaller in the subnanometer zeolitic pores than in the hydrated Nafion water channels of a few nanometers in diameter.8 The low resistance of the zeolite-T film can be attributed to its small d (5 mm) and enormous CH+ found by EDS analysis below. The zeolite-T membrane, after about eight days of operation in the VRFB electrolyte solutions for the experiments involved in this work, was cleaned using DI water and examined by SEM and EDS. Compared to the fresh membrane, the crystal size and shape were unchanged and the Si : Al ratio increased from B3.5 to B3.9 due to zeolite dealumination in acidic solutions. Meanwhile, the (Na + K) : Al ratio in the zeolite crystals decreased dramatically from B1.0 to 0.08. The EDS survey also showed a V:Al atomic ratio of 0.03. This minimal amount of vanadium was likely adsorbed on the external surface of the zeolite because the EDS line scan data showed the same level of vanadium content inside the alumina substrate. Thus, it can be concluded that more than 90% of the Na+ and K+ ions in the zeolite were exchanged with H+ in the acidic electrolyte solutions, resulting in a H+ : Al ratio of >0.9 in the zeolite. Based on the crystallographic structure of erionite9 (the primary phase in zeolite-T6), CH+ of the zeolite in VRFB electrolyte solutions is calculated to be B5100 mmol cm3, which is greater than that in Nafion 117 (1.6–3.2 mmol cm3)10 by three orders of magnitude. The polarization and charge–discharge curves of the zeolite membrane-equipped VRFB were examined at room temperature. The negative and positive electrolyte solutions were 10 ml of 2 M V2+/V3+ sulfate solution in 2 M H2SO4 and 10 ml of 2 M V5+/V4+ (VO2+/VO2+) Fig. 2 Ion concentration in the permeate side as a function of time during H+/V4+ (VO2+) diffusion measurement for the membranes. The H+/V4+ (VO2+) ion diffusion, electrochemical impedance spectroscopy (EIS), RFB polarization, and charge–discharge measure- ments were performed using a cell in which the electrolyte solutions flowed through 5 mm-wide gaps between the IEM and carbon felt electrodes mounted in parallel. The electrodes were simply con- nected by gold wires without current collecting plates. The EIS and charge–discharge tests were also carried for the commercial Nafions 117 film, a common benchmark IEM, for comparison. Fig. 2 presents the results of ion diffusion measurements using a feed solution of 4/7 M VOSO4 + 4/7 M H2SO4 and a permeate side solution of 1 M MgSO4 to balance the ionic strength and minimize osmotic pressure difference. The proton to V4+ (VO2+) permeation selectivity (aH/V) of the zeolite-T membrane was 87.5 which was much higher than that of Nafion 117 (aH/V = 16.8). The fluxes of H+ ( JH+) and V4+ ( JV4+) were 0.055 and 0.00063 mol m2 h1, respec- tively, for the zeolite-T membrane; and 0.54 and 0.032 mol m2 h1, respectively, for the Nafion 117 membrane. The porous a-alumina substrate alone has a small aH/V of 1.46 with JH+ and JV4+ of 0.168 and 0.115 mol m2 h1, respectively. The bare substrate exhibited a smaller JH+ than the Nafion 117 film because it had a small porosity (e = 0.3) and was much thicker (2000 mm) than the Nafion film (180 mm). The transport diffusivity of H+ in the Nafion 117 and porous a-alumina substrates obtained using Fick’s law was 2.36 1011 and 8.17 1011 m2 s1, respectively. The ion transport diffusivity of H+ and V4+ (VO2+) for the top layer of the zeolite, excluding the substrate resistance, was estimated to be B1.0 1013 and 1.54 1015 m2 s1, respectively. The JH+ and JV4+ of the zeolite layer without the substrate were then calculated to be 0.272 and 0.0021 mol m2 h1, respectively, which led to a aH/V of 130. When a solution of 2 M VOSO4 + 2 M H2SO4 was used in feed side, the zeolite membrane exhibited an actual aH/V of 120 with greater JH+ and JV4+ of 0.945 and 0.00787 mol m2 h1, respectively, because of increased ion transport driving forces. When the Nafion 117 film, the bare substrate, the supported zeolite-T membrane, and the Nafion 117 film attached to the substrate were, respectively, used as IEM, the total internal resistances of the cell measured by EIS were 4.74 O, 5.35 O, 5.73 O, and 5.74 O, respectively (Fig. 3). The Ohmic resistances of the zeolite layer, the Nafion 117 film, and the substrate are View Article Online Thisjournalis©TheRoyalSocietyofChemistry2014 Chem.Commun.,2014,50,2416--2419 | 2417 Published on 09 December 2013. Downloaded by Nanjing University of Technology on 18/04/2014 06:15:35.PDF Image | zeolite ion exchange membrane for redox flow batteries
PDF Search Title:
zeolite ion exchange membrane for redox flow batteriesOriginal File Name Searched:
zeolite-ion-flowbatteries.pdfDIY PDF Search: Google It | Yahoo | Bing
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
Heat Pumps CO2 ORC Heat Pump System Platform More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |